Introduction
Nitric oxide (NO) is an endogenous molecule released by endothelial cells, macrophages, liver cells and neurons [1]. NO is a free radical generated by three different nitric oxide synthase enzymes (NOS): inducible NOS (iNOS), neuronal NOS (nNOS) and endothelial NOS (eNOS) [2]. iNOS binds calmodulin and is highly implicated in immune functions as its expression is induced in inflammatory reactions by cytokines and endotoxins [3]. In addition, iNOS increases blood pressure and the progression of vascular dysfunction leading sometimes to harmful effects on the myocardium. eNOS is calcium-calmodulin controlled isoenzymes, producing NO with a vasodilator effect [4]. In cardiac pathogenesis, the NO system plays a dual role. While NO secreted by the eNOS system has a cardiac protective effect since it reduces left ventricular hypertrophy (LVH) [5] and cardiomyopathy [6], the NO secreted by the iNOS system initiates cardiac remodeling leading to LVH and cardiac fibrosis [7]. NO secreted by eNOS requires arginine and the bioavailability of this amino acid affects the synthesis mechanism [8]. The arginine is mostly produced in the organism where the kidneys play a key role in its synthesis, and the elimination of asymmetric dimethylarginine (ADMA), a metabolic by-product of continual protein modification processes [9]. ADMA is the result of arginine methylation which interferes with the NO synthase [10]. High levels of ADMA were often associated with CKD, oxidative stress, heart diseases, cardiovascular mortality in chronic kidney disease (CKD) patients and occasionally endothelial dysfunctions [11]. Therefore, reducing ADMA levels in CKD patients would improve their general outcomes. While it seems difficult to manipulate the ADMA level in the organism, different bioactive molecules were suggested to control the health issues associated with its increased level. For instance, angiotensin converting enzyme inhibitors (ACEI) are effective in controlling hypertension, a major risk factor for heart diseases and stroke, by inducing vascular relaxation and reducing blood volume [12]. Several studies confirmed that ACEI improve heart failure by decreasing preload, afterload and cardiac myocyte hypertrophy [13]. Interestingly, ACEI would interfere with the degradation of a vasodilatation peptide, bradykinin [14]. In addition, ACEI decrease proteinuria, preserve renal functions and slow the progression of renal disease [15]. Thus, mortality in symptomatic and asymptomatic patients with a left ventricular dysfunction are reduced by ACEI treatment [16]. Likewise, ascorbic acid (Vit C), a powerful antioxidant reduces the ADMA levels and associated complications, mainly oxidative stress and central blood pressure when it is used systemically in CKD patients [17]. To date, there has been no investigation into the potential benefits of administering both ACEI and Vit C in the management of cardiovascular diseases in CKD patients.
Therefore, the present study aims to analyze the efficiency of combining ACEI and ascorbic acid as a treatment for CKD patient with LVH.
Material and methods
This is a descriptive longitudinal study with prospective collection, involving 153 patients with chronic renal failure over a period of 2 years. As inclusion criteria, every patient aged over 18 years, with confirmed CKD of various origins and at different progression stages, including hemodialysis patients were considered. In addition, patients must be clinically stable for the 3 months prior to the onset of the study and may not receive treatment with injectable iron since it would affect the levels of oxygenated free radicals [18]. CKD patients with pre-existing heart diseases, severe valvular heart disease, constrictive pericarditis, systolic dysfunction with ejection fraction less than 50%, glomerular filtration rate (GFR) greater than 90 ml/min, and patients on peritoneal dialysis were excluded.
Included patients were classified into four stages according to the progression of CKD, mild (n = 31), moderate (n = 31), severe (n = 31) and terminal CKD stages (n = 60). The CKD stage was estimated based on the creatinine clearance, which was calculated by the MDRD formula [19]. Clinically healthy patients (n = 30) without any history of kidney and cardiovascular diseases were recruited as a control group (Table I). The number of patients in each group was estimated by calculating the number of the incidence of the pathology in the region.
Table I
General characteristics of each group
| Parameter | Mild CKD | Moderate CKD | Severe CKD | Terminal CKD | Controls |
|---|---|---|---|---|---|
| Number | 31 | 31 | 31 | 60 | 30 |
| Age | 65.7 ±10.4 | 56.0 ±13.9 | 59.1 ±9.9 | 54.6 ±6.8 | 34.3 ±7.8 |
| Hemoglobin [g/dl] | 11.5 ±2.29 | 11.3 ±1.10 | 9.78 ±0.96 | 9.98 ±0.90 | 13.3 ±0.76 |
| Cholesterol [mg/l] | 1.90 ±0.06 | 2.3 ±0.18 | 2.69 ±0.43 | 1.34 ±1.53 | 1.59 ±0.49 |
| Triglyceride [mg/l] | 1.51 ±0.81 | 2.55 ±1.54 | 2.15 ±1.33 | 2.79 ±1.34 | 1.7 ±0.73 |
| CRPus [mg/l] | 0.76 ±0.19 | 3.34 ±0.93 | 9.29 ±1.64 | 15.66 ±0.35 | 0.53 ±0.57 |
| Frequency of LVH | 18.78% | 23.3% | 34.8% | 45.7% | 0% |
| Dosage of iNOS [μmol/l] | 55.38 ±0.87• | 61.34 ±0.78•• | 64.08 ±0.45•• | 74.90 ±0.28••• | 50.7 ±0.02 |
| Dosage of ADMA [μmol/l] | 6.15 ±0.76•• | 15.03 ±0.9•• | 22.9 ±1.2••• | 32.03 ±0.78••• | 2.1 ±0.02 |
Blood samples were collected for iNOS-originated NO and ADMA measurement, in addition to general metabolic profiling by measuring hemoglobin, cholesterol, triglyceride and C-reactive protein – ultrasensitive (CRPus).
LVH was diagnosed in the studied patients using cardiac ultrasonography.
All patients with confirmed LVH received an oral treatment combining ACEI, Ramipril 5 mg, with ascorbic acid 200 mg/day during 2 years. Blood sampling for the measurement of iNOS-originated NO and ADMA was conducted before and after treatment. Cardiac ultrasonography for LVH progression was conducted after the end of the treatment protocol. Blood samples were collected in tubes containing ethylene diamine tetra acetic dipotassium acid (EDTA K2). The tubes were centrifuged for 10 min at 4500 rpm (3900 g), then aliquoted and kept in the freezer at –20°C until further analysis.
iNOS-originated NO measurement
The pro-oxidizing iNOS-originated NO was measured using a colorimetric method. NO was estimated based on the quantification of its two physiological metabolites; nitrites (NO2 –) and nitrates (NO3 –), according to Griess (1879) [20]. Nitrates were previously reduced to nitrites to be quantified. The concentration thus obtained represents the sum of nitrites and nitrates. NO levels were determined at the plasma level following deproteinization, using a solution of zinc sulfate (ZnSO4). Plasma deproteinization is necessary because turbidity due to the presence of proteins causes interference on the Griess reaction. Thus, falsely increased results are obtained if the sample is not deproteinized. To determine the total nitrites, each sample was incubated in the presence of 200 mg of cadmium and stirred for 10 min at room temperature. A volume of 400 ml of the mixture was added to 1500 ml of sulfanilic acid. After incubation for 10 min in the dark, 160 ml of N-naphthyl ethylenediamine was added. The mixture was incubated again for 10 min in the dark. The intensity of the staining was measured at 550 nm wavelength. The total nitrite concentration was determined by extrapolation of the value of the optical density (OD) readings on the standard curve DO = f sodium nitrite (NaNO2 –) previously established from the range of NaNO2 –. The normal value of the controls was 52.19 ±2.1 μmol/l.
ADMA measurement
ADMA plasma levels were measured by high-performance liquid chromatography (HPLC), using pre-column derivatization with o-phthalaldehyde (OPA), after extraction of plasma samples on solid phase extraction cartridges CBA (Varian) [21]. The coefficients of variation of this method were 5.2% for intra-assay and 5.5% for inter-assay; the detection limit of the assay was 0.1 μmol/l. Normal value in controls was 2.1 ±0.01 μmol/l.
Echocardiography
The clinical examination of heart function was performed using an echocardiograph (GE VIVID S6 Ultrasound Machine, KPI Healthcare Inc., 23865 Vía del Rio, Yorba Linda, CA 92887, USA) equipped with a 3.5 MHz probe and functional on time movement (TM), two-dimensional (2D) modes. Echocardiography was used to measure the diameter of the left ventricle and the thickness of its walls [22]. In addition, the left ventricular mass index (LVMI) was measured in linear M mode according to the following formula (according to ASE: American Society of Echocardiography Guidelines): LVMI = 0.8 × {1.04 [(LVIDd + PWTd + SWTd) 3 – (LVIDd) 3] } + 0.6 g, where LVIDd represents left ventricular internal diameter in diastole; PWTd is the posterior wall telediastolic and SWTd refers to the posterior septal wall telediastolic.
Left ventricular geometry was analyzed according to the ratio of DTIS/PWTd (diastolic thickness of the interventricular septum/diastolic thickness of the posterior wall) [23, 24].
Statistical analysis
The statistical analysis was performed using the Statistical Package of Social Sciences software “SPSS n° 25”. The comparison of the two averages was made by the student test. The comparison of more than two means of the continuous variables was made by the ANOVA test for parametric tests and for nonparametric tests, the Welch and Brown-Forsythe tests were used. Tukey and Hartmann test was used for multiple comparisons. The χ2 test was used for qualitative variables. For all tests, a p-value < 0.05 was considered significant. Pearson’s test was used for linear correlations. For multivariate studies of independent factors, we used a step-by-step Wald-type logistic regression model where all factors with a p > 0.1 were included in these analyses. The log rank test was used as a comparison test.
Results
The average age of the studied population was 58.85 ±12.75 years (Table I). The prevalence of LVH increased concomitantly with the degradation of the kidney function and the progression of the CKD where it was 18.78% for mild CKD, 23.3% for moderate CKD, 34.8% for severe CKD and 45.7% for terminal CKD. Likewise, iNOS increased gradually with the progression of the CKD stage.
In CKD patients, iNOS levels were significantly higher compared to the control group (Table I). The lowest values were reported in the mild stage (28.39 ±1.55 μmol/l), while the difference was not significant between moderate and severe stages (55.38 ±0.87 vs. 64.08 ±0.45 μmol/l). As expected, the highest values were recorded at the terminal stage (74.90 ±0.28 μmol/l). ADMA levels were the lowest in the control group, compared to CKD groups. Among CKD patients, the lowest ADMA values were recorded in mild and moderate stages (6.15 ±0.76 vs. CKD: 15.0 3±0.9 μmol/l, p > 0.05). The highest readings were recorded at the terminal stage but the difference was not significant as compared with severe stage (22.9 ±1.2 vs. 32.03 ±0.78 μmol/l, p > 0.05) (Table I).
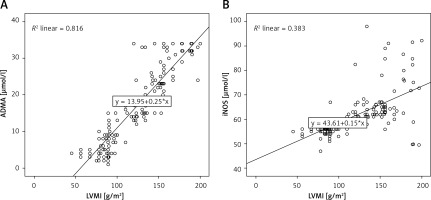
Negative and significant correlation between GFR in CKD patients (Figure 1) according to the different CKD stages and iNOS (–0.764, p = 0.0001) and ADMA (–0.948 and p = 0.0001) markers, as well as with the LVMI (–0.905, p = 0.0001) was observed. A positive significant correlation was found between LVMI and the two markers ADMA (0.901, p = 0001) and iNOS (0.718, p = 0.0001) (Figure 2).
Figure 1
Correlation between the glomerular filtration rate (MDRD) and nitric oxide generated by iducible nitric oxide synthase enzyme (iNOS) (A), asymmetric dimethylarginine (ADMA) (B) and left ventricular mass index (LVMI) (C) in patient with chromic kidney disease
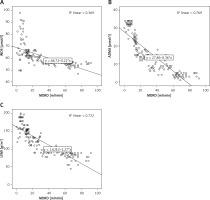
Figure 2
Correlation between left ventricular mass index (LVMI) and asymmetric dimethylarginine (ADMA) (A), and nitric oxide generated by iducible nitric oxide synthase enzyme (iNOS) (B) in patient with chronic kidney disease
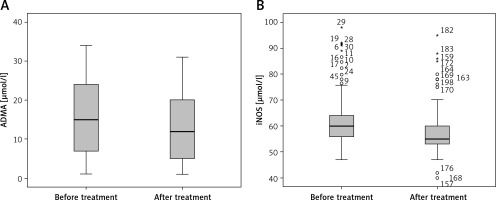
After 2 years of treatment for LVH using a combination of ACE and vitamin C, both markers iNOS and ADMA were decreased (p = 0.0001), compared to the levels before treatment. The same statement was reported for LVH (Figure 3).
Discussion
In the present study, NO secreted by iNOS increased concomitantly with the CKD stage, where the highest levels were recorded at terminal CKD. Reddy et al. [25] reported different findings where NO levels decreased in all CKD patients. This difference could be explained by the origin of the measured NO in the two studies. While in the present study, iNOS-originated NO was measured, due to its harmful effect on the cardiovascular system [26], Reddy et al. [25] measured NO originated from endothelial NO synthase (e-NOS), which is known for its benefits for the organism [27]. According to the latter study, the low levels of NO in CKD patients would be the result of low availability of L-arginine implicated in the biosynthesis of e-NOS [28] or the high levels of ADMA, a powerful NOS inhibitor [29, 30]. Schmidt et al. [31] demonstrated that the decrease in NO production induced by eNOS in CKD patients may contribute to hypertension and CKD progression.
Similar results were reported by Meenakshi and Agarwa in 2013 [32]. The study included 60 subjects with 30 controls and 30 CKD patients in hemodialysis where iNOS-induced NO was measured. The authors found that NO levels were higher in CKD patients at the terminal stage. This is due to the procedure with high levels of toxic uremia that leads to the stimulation of inducible NO synthase produced by cytokines [33]. At high concentrations, NO is a cytotoxic molecule responsible for dialysis complications and causes nitric stress playing the role of a highly reactive free radical [34].
ADMA levels increased with the progression of renal dysfunction. ADMA is a natural amino acid that circulates in the blood and is excreted in the urine. It is also a competitive inhibitor of nitric oxide synthase (NOS) [35, 36]. Several studies investigated the implication of kidneys in the elimination of ADMA and stated that the ADMA plasma levels generally increase by more than four times, sometimes up to ten times, in patients with end-stage renal disease [37–39], and are often associated with a higher risk of cardiovascular mortality [40, 41]. Like patients at end-stage renal disease, hemodialyzed patients typically have high ADMA levels, however kidney transplant patients have decreased ADMA and improved vascular endothelial function [42, 43]. This confirms the implication of kidney filtration in the elimination of the ADMA from the blood stream. Furthermore, elevated plasma levels of ADMA are considered an important biomarker of CKD complications and possible cardiovascular complication and mortality [44, 45].
Since the first description of ADMA as an endogenous inhibitor of NOS [46], two approaches have been adopted to answer the question of its biological importance. The first one aims to explore the relationship between circulating ADMA and associated diseases, while the second approach aims to investigate the possible causality relation of ADMA with kidney disease and cardiovascular complications [47]. The first established that pathological association was in relation with renal failure [48]. Since ADMA is excreted in the urine, it should accumulate gradually as kidney function deteriorates and as a result, inhibition of NOS would produce adverse effects in many different organ systems [49]. Without a doubt, ADMA fulfills many characteristics of a uremic toxin [50]. It is a guanidine, a product of protein metabolism, which accumulates in case of renal failure, and could be eliminated by dialysis [51]. Both approaches conclude that ADMA represents important risk factors for various cardiovascular diseases such as hypertension, coronary heart disease, atherosclerosis, pulmonary hypertension, atrial fibrillation, stroke and peripheral vascular disease [52]. In addition, ADMA improves the decoupling of NOS to produce reactive oxidative species (ROS) such as superoxide anion (O2 -) and peroxynitrite (ONOO-), which could further reduce the cardiovascular bioavailability of NO [53].
In the present study, the mean values of NO secreted by iNOS and ADMA had a positive correlation with LVMI. A Japanese study by Kamezak et al. published in 2014 involving 840 patients compared the level of NO secreted by the eNOS system in patients with and without left ventricular hypertrophy. It concluded that patients with left ventricular hypertrophy had lower NO values compared to patients without left ventricular hypertrophy (38.23 ±4.52 μmol/l, 21.36 ±2.36 μmol/l) [54]. Studies have already indicated that plasma levels of ADMA and NO are predictive of cardiovascular morbidity and mortality in renal failure.
Uremic patients are a population at a high cardiovascular risk. LVH is a major component of morbidity risk in these patients. The link between endothelial function and vascular hypertrophy is well demonstrated in hypertensive patients where endothelial dysfunction in these patients is particularly pronounced [55]. The heart and arterial system form an integrated unit that responds coherently to hemodynamic stimuli and the endothelium plays a central role in regulating cardiovascular remodeling [56]. London et al. demonstrated the correlation between cardiac and arterial remodeling in uremic patients [57]. The relationship between endothelial dysfunction and cardiovascular remodeling in end-stage CKD patients has been confirmed by several studies cited in the literature, suggesting that endothelial dysfunction may promote structural changes in the cardiovascular system in dialysis patients [58, 59].
CKD patients with confirmed LVH were treated with a combination of ACEI and Vit C for 2 years. A significant decrease in both levels of ADMA and NO, as well as on the LVH were recorded. ACEI increase eNOS expression and activity and decrease iNOS levels in the aorta and cardiac myocytes [60]. It seems that the circulating nitrite/nitrate, which are the terminal metabolites of nitric oxide, are significantly affected by ACEI [61]. Vit C prevents oxidative damage by trapping ROS and nitrogen reactivators [62]. A reduction in total Vit C concentration, especially the ascorbate form is mainly due to limited intake of potassium-rich foods by CKD patients under conservative treatment. Low Vit C plasma levels in CKD patients were associated with an increased risk of major cardiovascular complications [63]. Oral ascorbate (1–1.5 g/week) or parenteral ascorbate (300 mg/dialysis session) are suggested to balance subclinical impairment [64]. However, the opinions regarding Vit C supplementation are divergent. For instance, Kamgar et al. [65] reported that Vit C intake did not affect the markers of inflammation, malnutrition and oxidative stress. Another randomized, double-blind clinical trial conducted by Singer [66] on Vit C supplementation (250 mg, three times a week) in CKD patients with 4th and 5th stage and hemodialyzed patients, demonstrated no benefit on preventing cardiovascular complications. However, other studies confirmed the beneficial effect of Vit C supplementation (200 mg/day) on the nutritional status of patients [67–69].
The main limitation of this study is the size of the sample; therefore, the generated data should be carefully used in a general population.
In conclusion, cardiac complications are a leading cause of mortality in chronic renal failure patients. Oxidative stress is a recognized risk factor for non-traditional cardiovascular complications. NO produced by the iNOS system triggers cardiac remodeling and leads to left ventricular hypertrophy and cardiac fibrosis. ADMA levels increase with the degradation of renal function. The present study confirmed that patients with a lower renal function exhibited higher levels of iNOS and ADMA, which were correlated with an increase in LVMI. However, treatment with a combination of vitamin C therapy and an ACEIresulted in a reduction in iNOS and ADMA levels, as well as a decrease in left ventricular hypertrophy in these patients.