Introduction
Late endovascular aneurysm repair (EVAR) failure may lead to sac pressurization, expansion, and eventual aneurysm rupture [1–3]. Late rupture is defined as rupture occurring more than 30 days after the EVAR procedure. It is a rare complication with an increasing incidence worldwide mainly due to the widespread application of EVAR [4–7]. However, it remains a devastating event, like a de novo abdominal aortic aneurysm (AAA) rupture as it carries a mortality rate ranging from 20% to 60% in recent studies [8–43]. Thus, the fundamental aim of EVAR which is protection from rupture is not fulfilled as 0.5% to 6% of EVAR patients have experienced rupture so far [44].
The aim of this study is to present updated information on post-EVAR late aortic rupture (LAR), which increases steadily worldwide as the data in the literature are limited. It comprises a current literature review and meta-analysis based on the recent evidence investigating the incidence, causes, treatment outcomes and prognosis of post-EVAR LAR.
Material and methods
A meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Rupture of the AAA > 30 days after the initial EVAR is defined as ‘late rupture’ and this was confirmed on imaging studies that revealed blood outside the aneurysm sac or on open conversion [14].
Information sources and search strategy
Studies were identified by searching electronic databases and scanning bibliographic references of articles. The National Library of Medicine’s Medline database was searched using the PubMed interface from 1991 to the present date. The last search was run on April 1, 2023. The databases were searched with an unrestricted search strategy, applying exploded Medical Subject Headings (MeSH) and keywords combined with the Boolean operators AND, OR and NOT to retrieve relevant reports. A second-level search included a manual screen of the reference lists of the articles identified through the electronic search. Eligibility assessment was performed independently in an unblinded standardized manner by 2 reviewers (S.P. and K.M.); disagreements between reviewers were resolved by consensus. The search identified 966 records. Studies were screened for bias and consistency by 2 authors. A 3rd senior surgeon resolved all discrepancies, and the included studies have all passed the quality and bias assessment. The literature search strategy is outlined in a study flow diagram (Figure 1, Table I). Fifteen articles reporting a total of 398 patients with late AAA rupture after EVAR fulfilled the inclusion criteria (Table II). The selected studies were published between 2000 and 2023, reflecting study periods extending from 1992 to 2022 [5, 14, 32–59].
Table I
Search strategy
Table II
Included studies according to study period, demographics, number of patients, and incidence of rupture after EVAR and time to rupture
Eligibility, exclusion and inclusion criteria
Cohort studies reporting late rupture after EVAR and case series including at least 6 patients were included in the study as this disease is relatively rare and this cutoff point was considered numerically significant but also relatively clinically relevant. Articles in languages other than English, case reports and small case series with less than 6 patients were excluded. Sporadic post-EVAR LARs included in observational studies primarily reporting clinical effectiveness of EVAR, elective open conversions or treatment of ruptured AAAs were considered ineligible due to inadequate relevant information. Patients with post-EVAR rupture of any age and sex and any type of stent-graft were included. Patients with symptomatic aneurysms without rupture were excluded. Patients with complex EVAR like chimney (chEVAR), fenestrated (fEVAR) or branched (bEVAR) were not included as our study was focused on the standard infrarenal cases.
Collection of data
Year of publication of each study, study period, demographic data, and clinical characteristics such as hemodynamic stability at presentation, type of stent-graft initially implanted, causes of rupture, time to rupture after initial EVAR, previous secondary interventions and compliance with post-EVAR surveillance were retrieved and analyzed. In addition, the type of treatment, mortality and morbidity rate according to the type of reconstruction and overall mortality were assessed.
Analysis of data and statistical analysis
Standard descriptive statistics (reported as means ± standard deviation (SD)) were used to summarize the demographic data of the recruited patients from all eligible studies. Proportions were used for estimation and expression of clinical variables. The primary endpoints of the meta-analysis consisted of in-hospital mortality and the comparison of mortalities between open repair and endovascular. The meta-analysis was conducted, in accordance with the recommendations of the Meta-analysis of Observational Studies in Epidemiology (MOOSE) group, of the two cohorts. The pooled proportion was calculated as the back-transformation of the weighted mean of the transformed proportions by using the random effects model proposed by DerSimonian-Laird. Heterogeneity among studies was estimated by using the χ2 test and the Cochran Q score (reported as I2 and representing the percent value of the heterogeneity). Funnel plots were constructed, and the identified extreme studies were excluded to increase the robustness of our analyses. Frequency study-specific estimates were pooled and are reported as proportions with 95% confidence intervals (CI). The meta-analysis and the calculation assessment were carried out using the Comprehensive Meta-analysis Version 4 (Biostat, Englewood, NJ, USA) statistical software.
Results
Clinical characteristics and treatment details
The study characteristics at the time of presentation of AAA rupture are outlined in Table II. The vast majority was men (86.4%), the mean age of all patients was 76.9 ±2.43. The number of cases in the included studies ranged from 6 to 86 patients. All reports contained a total of 398 ruptures. Twelve case series studies reported a total number of 27364 EVARs performed over the study period giving an incidence of 1.11% (95% CI: 0.77 to 1.05). The total number of ruptured AAAs over the study period, including de novo ruptures, was reported in only 5 studies. The proportion of late ruptures after EVAR to the total number of ruptured AAA was 12.4% (78/628 patients). The mean time to rupture ranged from 16 to 72.3 months (mean: 43.22 ±16.25) and the mean maximum aneurysm diameter at admission ranged from 6.4 to 9.5 cm (mean: 7.54 ±1.13 cm). The types of aortic stent-grafts used in the initial procedure are presented in Table III.
Table III
Types of stent-grafts used in the studies included in our meta-analysis
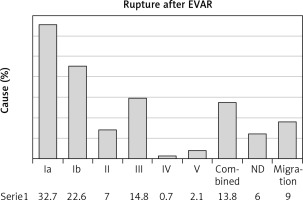
The reported reasons for rupture, as identified intraoperatively or on preoperative computed tomography, are outlined in Figure 2. Type Ia EL was the cause for rupture in 93 (32.7%) patients, while type Ib EL was the cause in 64 (22.6%) patients. Type III EL was the cause of rupture after EVAR in 42 (14.8%) patients, whereas type II EL was specified as the cause of rupture in 20 (7%) patients. Combined ELs were documented in 39 (13.8%) patients. Migration was encountered in 31 (9%) patients. The reason for aneurysm rupture was documented as “could not be determined” on 17 (6%) instances.
In 7 series reporting previous secondary endovascular interventions, nearly half of patients (56.7%) presenting with aneurysm rupture, had at least one such intervention following the initial EVAR procedure. Furthermore, in 13 series reporting patient compliance, nearly one quarter of patients were reported to have missed at least one EVAR surveillance appointment before rupture occurred (26.4%). Hemodynamic instability at admission was not mentioned in all series. 34.8% (69/198) of the patients with ruptured AAAs (in the series reporting instability) were hemodynamically unstable at presentation (Table IV).
Table IV
Characteristics and previous interventions and compliance to follow-up of patients included in our study
Of the 398 patients, 342 (85.9%, 95% CI 78 to 89) underwent interventional treatment: 190 (55.5%) open conversion, 149 (43.6%) endovascular repair and 3 (0.9%) hybrid treatment (aorto-uni-iliac graft with femoro-femoral bypass). The remaining 56 patients were managed with palliative care or died before reaching the operating table. One of the last patients remained alive during the first 30 days (Table V). Interventions for post-EVAR rupture were the following: A) Endovascular treatment including (1) chimney or fenestrated repair to extend the proximal sealing zone, (2) relining with a new stent-graft, (3) implantation of aorto-uni-iliac grafts plus femoro-femoral bypass, (4) implantation of Palmaz stents, aortic cuffs or iliac extensions and (5) side branch or landing zone embolization. B) Open surgical conversion involving (1) total stent-graft explantation and interposition of a standard tube or bifurcated graft along with sac plication, (2) partial proximal or distal stent-graft explantation and bridging with a standard graft along with sac plication, (3) oversewing of lumbar or IMA or a fabric tear from within the sac along with sac plication, (4) aneurysmal neck banding or ligation of lumbar or inferior mesenteric artery (IMA) or IIA without aneurysmal sac opening [5, 14, 32–43].
Table V
Mortality and morbidity rate in our analysis
Mortality rates and outcome
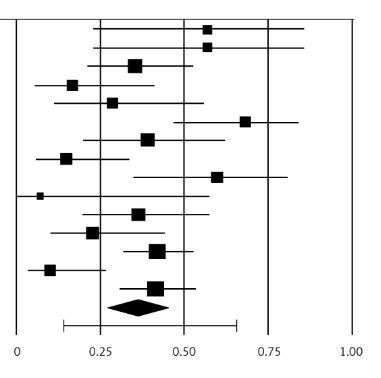
Treatment modalities and outcomes in the individual studies are presented in Table V. Meta-analysis of the case series studies revealed that the pooled estimate for in-hospital mortality was 35.6% (95% CI: 0.274 to 0.446, Figures 3, 4 A). Moderate heterogeneity was identified among the studies (The Q-value was 34.4, p = 0.002, I2 = 59%). Open surgical management was associated with a significantly higher perioperative mortality compared to endovascular intervention (pooled odds ratio 0.415, 95% CI: 0.207 to 0.831, Z-value is –2.483 with p = 0.013), (Figure 4 B). No significant heterogeneity was identified among the studies (The Q-value was 7.21, I2 = 0%).
Incidence of LAR
In our days, there has been stated that 1 out of 10 AAA ruptures arriving in an emergency department have a history of an antecedent AAA repair [25, 52]. This was also found in our study as the proportion of late ruptures after EVAR to the total number of ruptured AAAs was 12.4% (Tables II, IV). Post-EVAR LARs are far more common than late ruptures after a prior open repair [4, 43]. The incidence of post-EVAR LARs generally varies between 0.5% and 7% in many series [44]. There is a disparity in incidence mostly because a proportion of patients will die before reaching a hospital or will be presented to a different institution [8, 44]. Conversely, the risk of LAR after OR is much lower varying between 0.3% to 0.8% of patients within 3.0 to 5.5 years [5].
Generally, the risk of late aneurysm related death is < 3% in historic and modern studies, however, it is difficult to assess due to the uncertainty in cause of death registration and lack of adequate long-term cohorts [7]. EUROSTAR registry (European Collaborators on Stent/graft Techniques for aortic Aneurysm Repair) found a cumulative annual risk of rupture after EVAR of 2% at 6 years [59]. Our meta-analysis assessed a risk for LAR after EVAR of 1.11%. It varied between 0.18% and 3.1% in 12 case series, which reported the total number of EVARs performed over the study period. This rate agrees with other studies and with the results from two large databases. In Vascular Quality Initiative database, a 3% of 12,911 EVAR patients developed LAR at 5 years [24]. Moreover, in Medicare database, a 5.4% of EVAR patients developed LAR at 8 years [4].
Discussion
Timing of post-EVAR LAR
Our results indicate that post-EVAR LAR occurred between 16 and 72.3 months (mean: 43.22) (Table II). This agrees with other studies which state that the risk for LAR does not seem to decline over time, but it can occur at any time after the primary EVAR, with a peak at 3–-5 years [6, 44, 52, 54]. In contrast, LAR after OR needs more than a decade to happen and is usually caused by ruptured para-anastomotic aneurysms [43]. Rajendran et al. found an increase to time interval between EVAR and rupture (from 2.4 to 4.9 years) during the later years of the study which extended from 1992 to 2014 [42]. He attributed this to improvements in graft design, surgeon’s experience, follow-up rates, and increased life expectancy after the procedure [42]. On the contrary, Moulakakis et al. found a shortening of the time interval and attributed this partially to the fact that EVAR cases performed outside the manufacturer’s IFU or in patients with suboptimal anatomy, which have increased over time [8]. With the evolution of new-generation devices, larger studies are required to assess for potential reduction in post-EVAR ruptures [42].
Risk factors
Known risk factors for post-EVAR LAR are increased age, a large initial aneurysm size (> 6 cm), persisted sac expansion, a history of previous complications followed by endovascular secondary procedures and rupture as indication in the index operation [5, 53, 60]. In a case series the initial size was nearly 6 cm and size at rupture was nearly 8 cm while in another meta-analysis the size at rupture was 7 cm [42, 44]. Secondary interventions and incomplete follow-up are frequent prior to LAR, occurring in at least a third of patients, respectively [5, 44]. These agree with our meta-analysis which found that nearly one quarter of patients missed at least one EVAR surveillance appointment before rupture occurred (26.4%) and nearly half of them (56.7%) had previous secondary interventions (Table IV). We noted that different kinds of stent grafts had been used in primary AAA repair in patients with post-EVAR LAR, some of which have already been abandoned and others have been improved (Table III). In a recent report there was no significant difference in the post-EVAR rupture incidence rate between stent graft types and surprisingly, history of smoking was less frequent among post-EVAR rupture patients (60% vs. 82%; p < 0.001) [54].
Causes at rupture
The mean maximum aneurysm diameter at the time of rupture ranged from 6.4 to 9.5 cm (mean: 7.54 cm) in our study (Table II). We found that the most common intraoperative findings at the time of rupture were ELs type I (Ia or Ib in 55.3%) and III (in 14.8%) (Figure 2). Late type I and III ELs pressurize the sac which might had shrunk in the past and became thin; thus, new-onset pressurization may lead to rupture [8]. Generally, if left untreated these ELs may lead to moderate sac expansion and subsequent rupture. Consequently, once diagnosed, they must be treated promptly with endovascular or open repair [7, 44]. We found type II ELs in 7%. Type II ELs have a more benign course and are responsible for less than a tenth of late ruptures [16]. However, type II ELs may lead to sac expansion and graft distortion with subsequent graft related Els [28, 44]. We found endotension in 2.1% of post-EVAR LARs. Although endotension is a rare cause of LAR, rupture may affect up to 25% of endotension patients [61, 62]. Graft migration was found in 9% of post-EVAR LAR patients in our study (Figure 2).
Mortality after post-EVAR rupture vs. de novo rupture
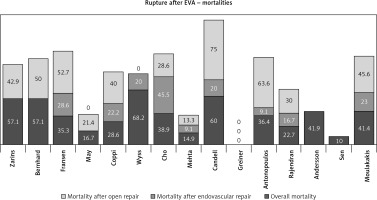
We found a pooled estimate for in-hospital mortality of 35.9% in our meta-analysis (Table V, Figure 3). It has been stated that patients with a post-EVAR rupture are more likely to present at the emergency department hemodynamically stable due to the protective effects of an existing intravascular stent-graft as massive exsanguination is hard to occur [35, 36, 42]. This would have a positive effect on patient outcome because stable patients show lower morbidity and mortality postoperatively. Unfortunately, this consideration does not agree with our study, where the mortality was substantial (35.9%). Other studies agree with these results [30, 38, 63]. Cho et al. found similar rates of hemodynamic stability and mortality between post-EVAR LAR patients and de novo ruptures [38]. Coppi et al. found a trend towards increased hemodynamic stability and mortality post-EVAR LAR patients but without statistical significance [36]. In contrast, in Rajendran and May reported series the proportion of unstable patients was significantly less after EVAR than after de novo ruptures (p < 0.01) [42]. Additionally, the difference in perioperative 30-day mortality rate (20% vs. 49%) was also significant (p < 0.01). Rajendran and May claim that de novo ruptures occur at a smaller AAA size (6.9 vs. 8.1 cm) than post-EVAR ruptures, which indicates decreased sac pressurization owing to the presence of the intravascular device [42]. Moreover, ruptures due to ELs may remain contained after thrombosis of the extravascular EL channel in contrast with de novo ruptures where the defect in the sac is unlikely to thrombose [42]. This belief is not confirmed in our meta-analysis which included a significant larger patient cohort and is consistent with recent literature data [43, 45]. Our results agree with a recent publication, which found a 30-day mortality of 41.4% independently of the presence of an intravascular device [8]. Additionally, in another recent publication with 60 ruptures after EVAR, which underwent interventional treatment (endovascular or OR), the overall mortality rate was 42% at 30 days [5]. A meta-analysis of 152 ruptures showed a pooled estimate for perioperative mortality of 32%, while some other studies have shown even higher mortality rates of up to 67% [14, 37, 41, 53]. Mortality was 56% in a series with 100 graft explantations due to rupture [31]. Additionally, in the Vascular Quality Initiative registry, mortality was 51.5% with open conversion for rupture compared with a 35.1% mortality for open primary ruptured AAA repair (p < 0.009) [64]. This mortality difference was attributed to a greater comorbidity burden in the open conversion patients and to the older age [30]. We must have in mind that the overall mortality rate across the three landmark randomized trials (IMPROVE, AJAX, ECAR) for ruptured AAAs was 32.6% [65–68]. Unfortunately, post-EVAR aneurysm-related mortality is increasing over time as has been reported in a recent meta-analysis of seven randomized trials [27]. Consequently, the assumption about the protective effects of the intravascular stent-graft needs further investigation.
Hemodynamic instability after post-EVAR rupture vs. de novo rupture
We found that nearly one third of patients (34.8%) presented at the emergency department with hemodynamic instability (the rate varied between 22% and 55.6% in 8 studies reporting unstable patients) (Table IV). In three of these studies, approximately one-third of patients are presented as unstable [8, 44, 69]. On the other hand, regarding the primary de novo ruptures, systematic reviews and meta-analyses, have shown an occurrence of instability between 28% and 48% [56, 70]. In a recent study hemodynamic status at presentation was the most important predictive factor of intraoperative and 30-day death, and hemodynamic instability was predictive of death in the patients treated either endovascularly or by open repair [8]. Previous EVAR and hemodynamic stability are independent predictors for improved mortality rates after rupture [42]. A stable patient offers the advantage of time to obtain a CT scan, allowing appropriate planning and thereby reducing postoperative complications and mortality [42].
Post-EVAR rupture treatment ‘endovascular vs. open repair’
Most LARs, regardless of the initial method of repair EVAR or OR, could be managed with endovascular techniques which are combined with lower morbidity and mortality [43]. We found lower mortality rates after EVAR than after OR in our study (19.8% vs. 39.7%, p = 0.013) (Table V, Figure 3, 4). Other meta-analyses have reported similar results. Antoniou et al. reported mortality rate after EVAR 21% vs. 37% with OR [44]. In recent series, Moulakakis et al. reported a mortality of 23.1% vs. 45.6% and Rajendran and May a mortality of 16.7% vs. 30% [8, 42]. Rajendran et al. and May et al. reported their results from the same center in Australia in consecutive time periods; their combined mortality was 12.5% for EVAR and 25% after OR [35, 42]. The profound causes are that the aortic clamping and the resulting physiological stress are obviated in EVAR instead of the majority of ORs. Additionally, OR is combined with greater blood loss [43].
Morbidity
We encountered postoperative complications ranging between 32% and 89% of post-EVAR LAR patients in the included studies (Table V). Fransen et al. reported complications in 32% (11 of 34 patients) [34]. These were, in brief, sepsis in 2 patients, acute renal failure in 5 patients, and access site hematoma or false aneurysm in 4 patients. Coppi et al. noted complications in 50% (7 of 14 patients): multi-organ failure in 3 patients, abdominal compartment syndrome in 1 patient and cardio-respiratory in 3 patients [36]. Cho et al. experienced complications in 66.7% (12 of 18 patients) [38]. Candell et al. reported complications in 89% (8 of 9 patients), cardio-respiratory in 6 patients, renal in 2, infectious in 3, moderate hematoma in 2 and multi-organ dysfunction in 1 patient [14]. Lastly, Sen et al. reported complications in 42% of their patients, renal in 3, cardio-respiratory in 6, bowel ischemia in 2 and return to the operating room in 3 patients [43].
Survival
In one recent study Sen et al. reported a survival of 76% at 1 year, 52% at 3 years and 41% at 5 years [43]. One year survival was reported to be 47% by Andersson et al., 20% Candell et al. and 27.8% Cho et al. [5, 14, 38]. In these older studies, survival seems to be inferior compared with survival after de novo ruptures.
Follow-up and surveillance
In our study we found that a quarter of patients (26.4%, ranging from 0% to 68.2% in the included studies) have not kept at least one recent follow-up appointment (Table IV). It is generally accepted that a significant proportion of post-EVAR LAR patients are noncompliant with surveillance protocols. In a recent publication, one in four patients with post-EVAR LAR lacked a recent scheduled surveillance [43]. Multiple failed reinterventions have been preceded and type I and III endoleaks predominate at the time of LAR. Many of these adverse events would have been treated if had been timely diagnosed at a regular surveillance appointment [13, 25, 43, 44, 59]. Consequently, improvement in surveillance compliance must be a main task of vascular facilities worldwide. It is noteworthy that aneurysm sac expansion or visible EL is not always present before post-EVAR rupture and ELs may not be detectable, even in cases with complete loss of seal. Consequently, their absence cannot exclude the risk of post-EVAR rupture [54]. Anatomic signs on follow-up CTA considered precursors of the subsequent post-EVAR rupture had been noted in 31% of cases before rupture and in 84% of cases, if reviewed retrospectively, using a structured protocol [54]. Patients with a ruptured AAA initially, need a more intense follow-up protocol as they present with LAR more often and earlier. Possibly, this is due to decreased IFU adherence caused by limitations in case planning and device availability [5]. It is recommended to perform a predischarge CTA in case of a ruptured AAA and select a high-risk group for more intense follow-up [54]. The radiation exposure should be taken into consideration, mostly for young patients [71, 72]. Additionally, surgeons must inform patients for the value of surveillance adherence and unwilling patients should be advised for alternative open surgery. After OR, a re-examination with a CTA of the entire aorta is recommended after 5 years according to 2019 ESVES guidelines [7].
EVAR vs. OR at the index procedure
One interesting issue raised by our study is the durability of EVAR. Randomized controlled trials, meta-analysis and real-world registry data have shown higher long-term all-cause mortality, higher reintervention rates, and secondary rupture rates after EVAR compared with open surgery [4, 6, 11, 53, 73]. Guidelines by the European Society for Vascular Surgery disclose that an open surgical first strategy should be recommended in younger fit patients with a long-life expectancy of more than 10 to 15 years (Class IIa, Level B) [7]. The National Institute for Health and Care Excellence (NICE) guidelines recommend not offering EVAR to people with an unruptured infrarenal AAA if open surgical repair is suitable [9]. This last recommendation has generated controversy and contention. In our opinion, EVAR should be offered with caution in young patients and in cases with aneurysm morphology incompatible with the manufacturer’s IFU.
Technical considerations
The approach for each post-EVAR LAR patient should be individualized, with the decision to choose between OC and EVAR depending on the patient’s fitness, hemodynamic stability and aortic anatomy [44]. Many of these complications can be treated by endovascular means urgently in a ruptured aneurysm or electively in an intact expanding aneurysm. Type I EL should be treated promptly to exclude the aneurysm from pressurized circulation. Endovascular options include graft balloon dilation, insertion of a bare metal stent or apposition of the stent graft fabric with endovascular staples (endoanchors) against the aortic wall, if the graft is adequately sized, has not migrated, and there is an appropriate landing zone to achieve a seal [7]. More commonly, extension of the landing zone is required with proximal tubular or fenestrated cuff insertion, or a branched repair to ensure a durable proximal seal, especially in those with aortic neck degeneration [52]. These innovations have reduced the need for open conversion for type Ia endoleak [30]. Distal seal can be achieved with iliac extenders [7]. Type II EL is treated with embolization and type III with relining [7]. EVAR is not an option when concern for infection is present [43]. Finally, if an endovascular solution is not available in reasonable time and the patient is fit, OC can be performed with acceptable results [7]. The technical approach to LAR with OC and an existing stent-graft device inside depends on the device design and the cause of rupture. We prefer a transabdominal approach. The initial endograft and any subsequent device placed later to treat endoleaks or migration may pose additional technical complexity. Proximal cuffs and fixation anchors may necessitate a more proximal clamping or a longer clamp time to complete the proximal anastomosis [30]. Usually, infrarenal clamping is possible only in cases of type Ia EL secondary to graft migration, such that a clamp zone was available between the renal arteries and the aneurysm [30]. Alternatively, aortic clamping along with the intraluminal graft can be performed. Regardless to the level of the clamping, suprarenal stents, hooks, or barbs can be left in place to avoid injury to the friable aortic wall and the renal arteries [30]. Sometimes, type Ia EL may be treated by external banding of the aneurysm neck. This requires infrarenal circumferential dissection and placement of a synthetic tight cuff around the neck of the aneurysm to restore the proximal seal. Sutures are placed through the external cuff, aortic wall, and endograft to reinforce the repair [30]. Treatment of a type II EL is feasible by sacotomy and surgical ligation of the lumbar or inferior mesenteric artery [30]. Although, endograft preservation may be preferred in high-risk patients, mortality remains significant [45]. Others believe that graft preservation is a lower risk procedure, alternative to graft explantation, with improved postoperative outcomes and good midterm durability, and should be considered in the management algorithm [30]. In addition, vascular surgeons must be familiar with the mechanisms of device failure and adequately trained to have advanced technical expertise and skills to perform a conversion to open repair, when necessary, especially in the emergent setting [29, 56, 74]. This may require dedicated open repair aortic workshops and training programs organized by medical societies or tertiary institutions 8.
Limitations: The study has limitations that should be considered when interpreting its results. Although multi-centered, the study reflects an elaboration of the retrospective and prospective data collected. As such, there may be differences in the quality of data collected. We do not have data on whether patients with de novo AAAs succumb more than patients with rupture after EVAR before reaching a hospital. A selection bias in choosing the operative approach, based on the suitable anatomy of the aneurysm or the hemodynamic status, might also exist. In general, for unstable patients, vascular surgeons often prefer open repair without delay for a CT scan. In recent years, occlusion balloons have been implemented in clinical practice. Compliance with follow-up protocol and secondary procedures was available in a limited number of patients before rupture. As was mentioned, most patients did not present their AAA rupture in the same institution where they were initially treated with EVAR. Overall, as the study contains real-world data, it can provide valuable information representing this surgical entity’s current status and treatment.
In conclusion, our analysis provided evidence that the most common causes of rupture after EVAR were type Ia and Ib ELs. Post-rupture mortality after EVAR was high (35.6%) and comparable to the morbidity of de novo ruptures. Endovascular repair appears to have better results compared to conversion to open repair. A significant number of patients had prior endovascular reoperations and inadequate follow-up. Patient compliance with the surveillance protocol is mandatory.