Introduction
Ketone bodies are endogenous metabolites produced by the liver, in particular under conditions of prolonged fasting, insulin deprivation, and extreme exercise [1]. Hepatic ketogenesis produces the three ketone bodies: acetone, acetoacetate (AcAc), and β-hydroxybutyrate (βOHB) acid [1]. Energy metabolism of the healthy heart depends mainly on the presence of free fatty acids and glucose, while ketone bodies and amino acids have a secondary role. Ketone bodies have a key role in energy metabolism of the failing heart. In the presence of heart failure (HF), energy metabolism is dependent on ketone bodies, mainly βOHB acid and acetone [2, 3]. Ketone bodies are an efficient substrate for cardiac metabolism, as they require less oxygen per molecule of adenosine triphosphate (ATP) generated. Recently, the cardioprotective effects of ketone bodies beyond energetics have been identified [2, 3].
The aim of the present review is to summarize the evidence and discuss the pleiotropic effects of ketone bodies on cardiac metabolism.
Ketone bodies’ metabolism
Ketogenesis is the biochemical process of the transformation, through lipolysis, of free fatty acids that are released from adipose tissue, into energy substrates [4]. Readily soluble in blood and water, concentrations of circulating ketone bodies are dynamic and they are oxidized in proportion to their delivery. They are, characteristically, produced in the liver in a fasting state, under a high-fat and low-carbohydrate diet (ketogenic diet), during prolonged exercise and at the time of diagnosis of type 1 diabetes (T1D) or during periods of poorly controlled T1D [1]. In the fasting state, fatty acids are mobilized and converted into ketone bodies by the liver, and then are transferred to peripheral tissues, where they undergo oxidation. Moreover, biosynthesis of ketone bodies is related to multiple metabolic pathways, such as the β-oxidation of fatty acids, the tricarboxylic acid cycle, sterol biosynthesis, de novo lipogenesis, glucose metabolism, and the mitochondrial electron transport chain [1, 4]. Therefore, the concentration of ketone bodies in the blood is dependent on nutritional and hormonal status.
Ketone bodies can be transported into the circulation without energy cost. They are able to freely pass the blood-brain barrier using the monocarboxylic acid transporters [5]. During conditions of oxidative stress liver ketogenesis is stimulated and high levels of ketone bodies are released into the circulation. Plasma ketone body concentrations in humans range from < 0.1 mM in a fed state up to 6 mM in a prolonged fasted state, and as high as 25 mM in diabetic ketoacidosis [6].
The human liver is able to produce up to 300 g of ketone bodies per day [7]. In non-fasting conditions, metabolism of ketone bodies provides about 5% of total energy requirements, an amount that is increased by approximately 20% under fasting conditions [8]. In the non-fasting state insulin inhibits ketogenesis via inhibition of hormone-sensitive lipase, thus preventing triacylglycerol breakdown to glycerol and fatty acids, and ultimately decreases the substrate for ketogenesis. Conversely, during a fasted state, adipose tissue lipolysis releases fatty acids for hepatic ketogenesis.
Oxidation of ketone bodies yields more ATP per g than glucose and therefore ketone bodies are classified as “super fuels” [9, 10]. In particular, the combustion of 100 g of acetoacetate or βOHB acid results in the production of 9,400 g and 10,500 g of ATP, respectively, whereas combustion of 100 g of glucose results in the production of 8,700 g of ATP. Ketone bodies are more energetically efficient fuels than other major energy substrates for the heart, particularly fatty acids and glucose [11]. βOHB acid contributes to a 24% improvement in cardiac efficiency, mitochondrial function, and the stabilization of cellular membrane potential, resulting in enhancement of the antiarrhythmic potential of the myocardial cell [12, 13]. The above was confirmed in a recent study in mice with hypertrophic hearts, which showed the shift of the energy homeostasis to ketone body consumption [14].
Ketogenic diet
Ketogenic diets (KD) are very low carbohydrate, high protein, very high fat content diets which induces ketogenesis (Table I). An increasing number of studies are exploring their possible therapeutic effects on many diseases. Intake of carbohydrates with consequent increase in insulin secretion inhibits lipolysis and promotes fat storage [4]. Conversely, the drastic reduction in carbohydrates to levels ≤ 50 g/day decreases circulating insulin, with the opposite effect. As the KD progresses, a gradual adjustment of metabolism is made to the new energy conditions. After 3–4 weeks the condition is similar to that observed in a fasting state [6].
Table I
Macronutrient concentrations of different types of ketogenic diet (% of total calories)
| Macronutrient substances ratio | Fat (%) | Protein (%) | Carbohydrates (%) |
|---|---|---|---|
| Classic KD | 90 | 6 | 4 |
| Modified KD | 87 | 10 | 3 |
| Modified KD | 82 | 12 | 6 |
| Modified KD | 70 | 15 | 15 |
| MCT (1.9 : 1) | *50/21 | 19 | 10 |
| Low glycemic index (2 : 3) | 60 | 28 | 12 |
| Modified Atkins diet | 65 | 29–32 | 3–6 |
Application of KD for weight loss dates from 1860, and was proposed as a treatment of choice in William Osler’s manual at the beginning of 1900. In the 1970s, KD became more widely known through the publication of the results of the extreme and not widely accepted Atkins diet [15]. However, over the past 15 years, many randomized studies and reviews have confirmed the relative safety at least in their short-term implementation, but also their good short- and medium-term effectiveness [16]. Based on the research data it appears that KD cause hunger reduction through saturation mechanisms from food proteins, effects on the hormones controlling appetite and direct action by ketone bodies [17].
KD have a favorable impact on the synthesis of saturated fatty acids, an established cardiovascular (CVD) risk factor, as well as on the levels of fasting triglycerides [18, 19]. KD have also been reported to show a significant decrease in blood pressure in overweight subjects [20, 21]. These data indicate that circulating ketone bodies derived from a KD might improve myocardium functioning and can contribute to the treatment of patients with impaired functions of the cardiovascular system [20, 21].
KD are considered to be “the most reliable example of dietary therapy with proven efficacy in certain neurological conditions” [22, 23]. KD and ketone bodies appear to play an important role in improving many neurodegenerative diseases, yet the neuroprotection mechanisms they exercise have not been fully elucidated. In addition to drug resistant epilepsy where a ketogenic diet has been shown to be effective, some reviews and studies also show a potential benefit in motor neuron disease, neurotrauma, brain injury, multiple sclerosis and migraine [23, 24].
At this point it must be mentioned that, on the other hand, literature data minimize the enthusiasm for KD. The use of KD for the treatment of pediatric epilepsy has been associated with adverse effects, ranging from fatigue, weakness, and gastrointestinal disturbances, to cardiac arrhythmias [25, 26]. Low-density lipoprotein cholesterol and apo-B-containing lipoprotein levels may fail to improve, or even significantly increase, with a KD despite weight loss [27]. In patients with T2D, a meta-analysis of randomized long-term studies comparing the KD with low-fat diets for weight loss showed no difference in glycemic control [28]. Finally, studies in animal models [29, 30] and children treated with KD [31, 32] have suggested retardation in skeletal development and reduction in bone mineral density.
Supply of ketone bodies to the heart
Cardiomyocytes are characterized by a high density of mitochondria that makes them capable of oxidizing various substrates to produce ATP [33]. However, whether or not the heart has ketogenic capacity is debatable. In adults, the heart consumes more energy than any other tissue (about 400 kcal/kg of myocardial tissue/day) [33]. As already mentioned, free fatty acids (in both non-esterified and esterified form) and carbohydrates (glucose and lactose) are the main sources of energy. It is noted that their oxidation represents about 90% of cardiac production of ATP. The metabolism of ketone bodies and branched chain amino acids is an alternative energy source for the myocardium [10, 34]. In particular, under regular feeding conditions, the main energy source of the cardiomyocytes is oxidation of free fatty acids, which provide about 60% of the requirements in ATP, while the remaining 40% of myocardial requirements in ATP are provided by glucose oxidation [10, 34]. However, in heart failure, there are increases in circulating levels of ketone bodies, mainly βOHB, along with up-regulated capacity of the failing heart to oxidize ketone bodies [10, 34].
Cardiac diseases are associated with loss of metabolic flexibility. Even in early stages of structural heart diseases, substrate utilization switches from fatty acids to glucose utilization, and oxidative metabolism is reduced [10, 34]. Under hypoxia, the main source of ATP is shifted from β-oxidation of fatty acids to glucose catabolism as the glycolysis may even function under anaerobic conditions (anaerobic glycolysis). However, as the output of ATP by this pathway is significantly lower than that of mitochondrial oxidative metabolism, more efficient sources of energy are required [10, 34].
Separate studies demonstrated that circulating ketone concentrations and cardiac ketone utilization are increased in a variety of clinical conditions, including HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction [35–38]. Ketone bodies are a favorable substrate of energy production as their conversion to acetyl coenzyme A is much easier compared to the corresponding conversion of free fatty acids and glucose [10, 34]. Plasma concentration of ketone bodies is increased in HF and is positively correlated with ventricular filling pressure [39]. Indeed, a positive correlation was found in patients with HF between the increase in myocardial energy costs and levels of βOHB acid and acetone, leading to the conclusion that ketone bodies could be used as bio-indicators in patients with HF [40]. Data from both human and experimental models confirm the use of ketone bodies as a cardiac energy substrate under ischemic conditions [38, 40].
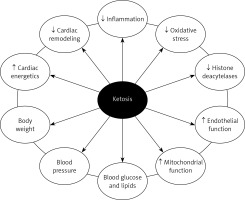
Increasing circulating ketone concentrations via KD or infusion of βOHB ameliorated pathological cardiac remodeling and improved cardiac function in small and large animal models of HF [41, 42]. The importance of the consumption of ketone bodies by the myocardium in HF has been demonstrated in an experimental model of Cre-loxBDH1 KO mice whose hearts did not have the oxidation capacity of βOHB acid (and not acetoacetate acid) [41]. Mouse BDH1-CO hearts showed increased fasting stress and pathological cardiac reflow after pressure overload/ischemic damage (aortic desquamation/myocardial infarction) compared to mouse hearts expressing the BDH1 gene and able to use βOHB acid. Pathological cardiac remodeling in response to pressure overload was also more pronounced in mice with cardiomyocyte-specific knockout of succinyl-CoA:3-ketoacid-CoA transferase [42]. It has also been shown that ketone bodies improve cardiac function with reduction in pathological cardiac remodeling in animal models of HF, associated with increased markers of myocardial uptake and oxidation of ketones [43]. Plasma βOHB and cardiac utilization are also increased in patients with diabetes and arrhythmogenic cardiomyopathy, suggesting that the ketogenic shift is the cardiac response to stress [44, 45] (Figure 1).
Ketone bodies and diabetic heart
Diabetic patients suffering from HF have been observed to have an increased cardiac uptake of ketone bodies as compared to those without diabetes [45]. Chronic insulin resistance in the diabetic heart results in alterations of fuel availability and change of the affinity and utilization abilities of myocytes for different substrates [46], with free fatty acids becoming the preferred substrate, which leads to significant reduction of energy efficiency and accumulation of toxic byproducts that exacerbate HF and insulin resistance [47]. Mizuno et al. [45] demonstrated that in diabetic HF the uptake of total ketone bodies is higher than in nondiabetic HF. This suggests that ketone bodies serve as a partial energy source replacement in the human diabetic heart.
Ketosis is a common finding in T1D, especially at the diagnosis of the disease. Therefore, increased plasma concentrations of ketone bodies in patients with T1D entail an increase in tissue availability, including the heart. In addition, in diabetes an increase in free fatty acids is observed as a result of increased lipolysis in fat tissue. Given the competition between these energy substrates [48], it is not entirely clear which dominates cardiac metabolism in the presence of diabetes. There is, however, significant evidence that the consumption of free fatty acids is increasing in the diabetic heart, at the expense of glucose [49] intake, and it is even estimated that the use of free myocardial fatty acids accounts for > 90% of the ATP requirements by the diabetic myocardium [50]. These result in steatosis and oxidative stress [51] of the diabetic myocardium, which are the main causative factors for diastolic heart dysfunction and ultimately diabetic cardiomyopathy. In contrast, it has been suggested that the increased consumption of ketone bodies from the heart in diabetes represents an adaptive mechanism aimed at better cardiac function.
SGLT2 inhibitors are a new class of antidiabetic agents which, in addition to reducing plasma glucose levels, have significant pleiotropic effects. Inhibition of SGLT2 results in glycosuria, which thereby lowers plasma glucose levels, resulting in a decrease in insulin levels and an increase in the levels of glucagon in the fasting state [44, 45]. The results of large clinical trials have shown favorable effects of SGLT2 inhibitors on cardiovascular and renal outcomes [52, 53]. One of the possible pathogenetic mechanisms explaining these effects is the mild ketosis caused by SGLT2 inhibitor therapy [11]. SGLT2 inhibitors increase the production of ketone bodies in the liver by increasing glucagon levels and decreasing the levels of plasma insulin [11]. Is seems that mild ketosis acts like a fuel for the failing heart and, therefore, therapy with SGLT2 inhibitors in diabetic patients with HF might improve cardiac energetics and cardiac efficiency [11].
Conclusions
Ketone bodies are not only products of metabolism but they also have a key role in providing energy to vital organs, such as the brain and heart, in both normal and pathological conditions. Only recently, based on the results of large clinical trials with newer antidiabetic agents, the role of ketone bodies in supplying energy in myocardial failure conditions was better understood. Therefore, new prospects are opening with ketone bodies playing a key role in the management of heart diseases as energy substrates.