Introduction
Atherosclerotic diseases, including stroke and acute coronary syndromes, are the leading causes of both mortality and disability in industrialized countries [1]. In the event of ST elevation myocardial infarction (STEMI), a large proportion of myocardium is usually jeopardized and ongoing complete interruption of the major coronary artery flow may lead to significant transmural scarring with a subsequent decrease of cardiac output. Primary percutaneous coronary intervention (PCI) is the preferred method of reperfusion in such occasions [2, 3]. Since atherosclerosis is a diffuse process, one is not surprised that up to 50% of STEMI patients have multivessel coronary artery disease (CAD), universally defined as ≥ 50% stenosis of at least one major epicardial non-infarct-related artery (IRA) [4, 5]. Whether to routinely revascularize these non-culprit lesions or to manage them conservatively with guideline-based optimal medical therapy alone is an ongoing dilemma as they may represent stable coronary artery plaques with a questionable benefit of additional revascularization. Several randomized studies have evaluated the potential benefit of complete revascularization with overall inconclusive results [6–10]. However, these studies may be (among others) limited by suboptimal evaluation of stenosis significance and, thereby poor stenosis selection strategy. In this single-center study, we provide the correlation of angiographic estimation of stenosis severity and hemodynamic measurement of the significance of non-infarct-related residual stenoses in such patients.
Material and methods
Study design
All adult patients with acute STEMI as the primary manifestation of CAD who had undergone successful PCI (Thrombolysis In Myocardial Infarction “TIMI” score at least 2) of the culprit lesion were candidates for enrollment. The major inclusion criterion was at least one residual stenosis in non-infarct-related major coronary artery (diameter ≥ 2.5 mm). The key exclusion criteria are summarized in Table I. All patients gave written informed consent to the study protocol. The study was performed in accordance with the Declaration of Helsinki of the World Medical Association and the local ethics committee approved the study protocol.
Table I
Key exclusion criteria
Angiographic evaluation of stenosis severity
Following successful PCI, the primary operator evaluated the severity of residual disease based on visual estimation including a summary of percentage of stenosis in each eligible lesion. Moreover, the operator was asked to estimate the likelihood of positivity of the following hemodynamic evaluation of the non-IRA (“yes/no” question) as well as to underline the relevant indicators of such choice in multiple-choice question (stenosis severity ± diameter of the arterial segment ± localization/proximity of the lesion ± estimated size of the distal vascular bed). Such an approach better reflects lesion selection in routine clinical practice and does not rely solely on percentage of stenosis. All patients were discharged with guideline-recommended optimal medical therapy.
Hemodynamic evaluation of stenosis severity and completion of revascularization
The invasive hemodynamic evaluation of all eligible non-IRA lesions was performed during subsequent elective hospitalization within a period of 4–8 weeks after primary PCI as is the current standard in our institution. The hemodynamic measurement was performed using fractional flow reserve (FFR) – an invasive technique evaluating the hemodynamic relevance of coronary stenosis by means of the measurement of the relative poststenotic pressure drop during maximal coronary vasodilation. It is currently considered the most direct way to assess the hemodynamic significance of individual coronary lesions and is recommended in all patients with borderline stenosis without non-invasive measurement of the extent of ischemia [11]. All operators were experienced in its use including standardized application of adenosine as well as the use of dedicated pressure wire (PressureWire AERIS™, Abbott). An FFR value of 0.80 or less following maximal vasodilation was considered to be significant and requiring revascularization. The method of final revascularization was based on the operator’s recommendation – either coronary artery bypass graft procedure or PCI with a strong recommendation for the use of drug eluting stents.
Statistical analysis
Standard descriptive statistics were used to describe the study group. Categorical variables were reported as numbers and relative frequencies (percentages) and continuous variables as the mean ± standard deviation. Logistic regression analysis and receiver operating characteristic analysis were applied with p-value < 0.05 considered statistically significant.
Results
A total of 51 patients (aged 62.7 ±10.2 years) were enrolled from November 2020 to September 2022. Their baseline characteristics are summarized in Table II. In this study group, a total of 65 stenoses (67.9 ±10.7% stenosis, 2.98 ±0.32 mm diameter) were recommended for subsequent hemodynamic measurement – 39 patients with single-vessel disease, 10 patients with two-vessel disease and 2 patients with three-vessel disease. Based on angiographic evaluation, a total of 44 stenoses (67.7%) would be recommended for treatment (70.6 ±10.6% stenosis) whereas the rest of the stenoses were considered non-significant (62.4 ±9.0% stenosis) and thus would be recommended for a further conservative approach.
Table II
Patients’ baseline characteristics
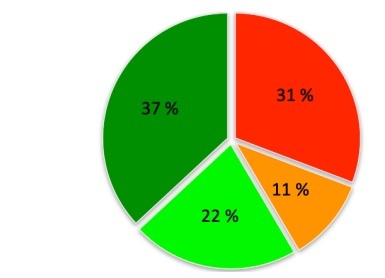
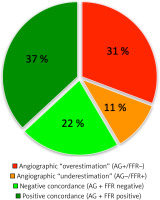
Subsequently, all study patients underwent successful hemodynamic measurement of all eligible stenoses. No clinical adverse cardiac event was reported within the time period from discharge to subsequent hospitalization. Compared to angiographic measurement, a total of 31 (47.7%) stenoses were considered hemodynamically significant and eventually recommended for treatment (71.8 ±11.6% stenosis, average FFR value 0.69 ±0.08, FFR range: 0.51–0.79). The remaining 34 measurements were negative (64.4 ±8.5% stenosis, average FFR value 0.87 ±0.04, FFR range: 0.81–0.96). In summary, less revascularization procedures were recommended based on FFR measurement compared to angiographic estimation.
Interestingly, based on individual comparison, 27 of 65 measurements (41.5%) were discrepant when comparing angiographic to hemodynamic measurements (Figures 1 and 2). In summary, 24 of the 65 stenoses were considered angiographically positive and were recommended for revascularization based on FFR measurement, 14 stenoses were confirmed to be negative as estimated, 20 stenoses were estimated positive but left for a conservative approach based on FFR measurement and, finally, 7 stenoses were estimated non-significant but eventually revascularized based on FFR measurement. The mean percentage values within individual subgroups are summarized in Table III. Importantly, when related to FFR (a current gold standard of invasive measurement), visual estimation would provide the overall sensitivity of 77.4% but the specificity of 41.2%. The false positivity and negativity rates would be 58.8% and 22.6%, respectively.
Figure 2
Distribution of individual percentage stenosis values with relationship to angiographic and FFR concordance/discordance
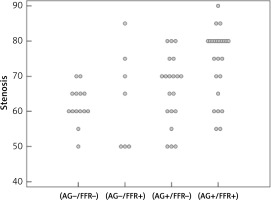
Table III
Mean percentage stenosis values
Regarding the operator’s decision-making process, of the four offered indicators (multiple-choice) the most frequently considered relevant was the estimation of the size of the supplied territory (66.2%) closely followed by the stenosis severity (64.6%). On the other hand, the localization of the stenosis (proximity) and the caliber of the stenosed segment played less significant roles (35.3% and 20%, respectively). Table IV summarizes the distribution of indicators with relation to angiographic estimation of the stenosis significance (Table IV A) and subsequent angiographic and hemodynamic concordance (Table IV B).
Table IV
A – Proportional representation of morphological indicators in angiographic estimation of stenosis significance. B – Proportional representation of morphological indicators in relation to angiographic and FFR concordance/discordance
Moreover, angiographic estimations and subsequent concordance were evaluated in relationship to the left anterior descending (LAD) coronary artery territory compared to all other arteries/major branches. Overall, 29 out of 35 LAD stenoses (82.8%) were estimated significant with 62.9% finally FFR positive. On the other hand, non-LAD arteries/branches were estimated significant in 15 of 30 stenoses (50.0%), of which 30% were FFR positive. The discordance rates in LAD vs. non-LAD territory were 31.4% and 53.3%, respectively. Finally, when compared with the relationship to the severity of stenosis, in the 50–70% stenosis range the discordance rate was 47.7%, whereas in the 71-90% stenosis range the discordance rate was 30.4%. Using regression analysis, both LAD (p = 0.01, odds ratio = 3.949) and > 70% stenosis (p = 0.001, odds ratio = 8.031) were significant predictors of positive FFR conversely to stenosis caliber (p = 0.468, odds ratio = 0.553) or the operator’s visual estimation in general (p = 0.113, odds ratio = 2.4). The optimal cut-off value of stenosis severity for the prediction of positive FFR value was 72.5% with sensitivity of 58.1% and specificity of 85.3%.
Discussion
It is notoriously known and well documented that up to 50% of patients with STEMI have significant (≥ 50%) residual stenoses exclusive of an IRA [4, 5]. Historically, it was recommended to conservatively treat such lesions due to the increased rate of adverse events, including mortality [12–14]. However, with increased safety and efficacy of PCI procedures, there has been a shift in the paradigm and, according to the current guidelines, it is recommended that the revascularization is completed in such patients [3]. There are, however, many unanswered questions.
In our study, we focused on STEMI patients as a primary manifestation of CAD excluding those with a previous history of acute coronary syndrome or stable CAD. As such, the finding of residual stenosis could be considered incidental and likely stable. The current guidelines on chronic (stable) CAD suggest that in the event of anginal symptoms absence and lack of non-invasive documentation of the presence of ischemia, coronary stenoses in the range of 50–90% should only be treated in case of a documented FFR value of 0.8 or less [11]. These data are supported by the results of the FAME 2 trial suggesting that FFR guided PCI decreased the incidence of further urgent revascularization (but not the incidence of death from any cause or myocardial infarction) at a mean of 7 months, whereas the 5-year follow-up showed only marginal evidence of a decrease in the incidence of myocardial infarction [15]. Moreover, a large randomized ISCHEMIA trial published in 2020, which enrolled more than 5,000 patients followed over a median of 3.2 years has shown that among patients with stable CAD and moderate to severe ischemia, the initial invasive strategy as compared with an initial conservative approach did not reduce the risk of ischemic cardiovascular events or death from any cause [16]. There are, thereby, limited data supporting the mortality benefit of revascularization in patients with stable CAD excluding selected patient subgroups such as those with large (> 10%) ischemia on myocardial perfusion imaging or those with angiographic evidence of high-risk features such as significant (≥ 50%) left main stenosis [16, 17].
Several studies have specifically focused on the outcomes of STEMI patients with significant residual stenoses comparing an initial invasive to initial conservative strategy. The pioneering randomized trials each enrolling up to hundreds of patients include PRAMI, CvLPRIT, DANAMI-3 PRIMULTI and COMPARE-ACUTE [6–9] which are summarized in detail within Table V. It is important to highlight that these studies differed significantly in several attributes, such as length of follow-up (12–27 months), timing of revascularization completion and, most importantly, lesion severity criteria (≥ 50%, ≥ 70% or FFR guided). In summary, the trials have shown a lower rate of adverse outcomes in initially invasive strategy driven by a decreased need for future revascularizations with only a numerical trend towards fewer non-fatal myocardial infarctions and no difference in mortality. Complete Revascularization with Multivessel PCI for Myocardial Infarction (COMPLETE) was a long-awaited large-scale trial published in 2019 [10]. Overall, 4,041 patients with STEMI and multivessel CAD were randomized following culprit lesion PCI to either no further revascularization or complete revascularization of all further significant non-culprit lesions irrespective of stable CAD symptoms prior to STEMI. The significance of the residual lesion was defined as either ≥ 70% stenosis based on angiography or 50–69% stenosis with FFR value ≤ 0.80. The main exclusion criteria were an intention to revascularize a non-culprit lesion before randomization or previous or planned coronary artery bypass grafting surgery. Revascularizations of non-IRA lesions were either performed during or after the index hospitalization. At a median follow-up of 3 years, there was a 2.7% absolute reduction in the coprimary outcome of cardiovascular death and new myocardial infarction, as well as 7.8% absolute reduction in cardiovascular death, new myocardial infarction and ischemia-driven revascularization in the complete revascularization group. The outcome was consistent across both stratified subgroups (complete revascularization during the index hospitalization or within 45 days of randomization). Like previous smaller scale trials, the COMPLETE trial also did not include patients in cardiogenic shock, limiting generalizability to that population. Despite the COMPLETE trial large sample confirmation that complete revascularization was associated with a reduction in ‘hard outcomes’ as the primary outcome was cardiovascular death or myocardial infarction, the result was primarily driven by the lower incidence of new myocardial infarction (non-STEMI of uncertain significance defined by increase of cardiac markers; no difference in STEMI) in favor of complete revascularization (5.4% vs. 7.9%), with no significant difference in the incidence of death from cardiovascular causes (2.9% vs. 3.2%, respectively).
Table V
Overview of major randomized controlled trials comparing culprit only to complete revascularization in acute STEMI patients
Because estimation of stenosis significance based solely on stenosis severity is known to likely overestimate its hemodynamic relevance [18], and keeping in mind that the common angiographic definition for the residual stenosis is quite unrestrictive (≥ 50% in any artery with a diameter greater than 2–2.5 mm), we decided to evaluate the accuracy of visual estimation to predict the hemodynamic significance with respect to further morphologic attributes commonly used in daily clinical practice. Apart from stenosis severity, such attributes were the diameter of the stenosed segment, location/proximity of the lesion and the estimated size of the vascular bed. We found that even under such circumstances, the inaccuracy rate exceeds 40% with increased tendency towards overestimation, particularly in non-LAD territory and in stenoses with 50–70% range. All despite being a high-volume PCI center with free access to and a large experience with FFR measurement. Interestingly, a Multivessel PCI Guided by FFR or Angiography for Myocardial Infarction (FLOWER-MI) trial focusing on patients with STEMI was published in 2021 [19]. The authors randomly assigned patients with residual multivessel CAD to receive either complete revascularization guided by FFR or complete revascularization guided by angiography (≥ 50% stenosis). The primary outcome was a composite of death from any cause, non-fatal myocardial infarction or unplanned hospitalization leading to urgent revascularization at 1 year. Completion of revascularization was strongly recommended to be performed as early as possible after primary PCI. However, the staged intervention for non-culprit lesion was used in more than 95% of the patients in each group, with the mean time delay between the intervention being 2.6 ±1.4 days in the FFR-guided group and 2.7 ±3.3 days in the angiography guided group likely reflecting the low tendency for immediate complete revascularization in routine clinical practice. The mean number of stents used per patient for non-culprit lesions was 1.01 ±0.99 in the FFR-guided group, and 1.50 ±0.86 in the angiography-guided group, which is consistent with our finding of a more restrictive approach to revascularization with FFR. The primary outcome event occurred in 32 of 586 patients (5.5%) in the FFR-guided group and in 24 of 577 patients (4.2%) in the angiography-guided group. Death occurred in 9 (1.5%) patients in the FFR-guided group and in 10 (1.7%) patients in the angiography-guided group; non-fatal myocardial infarction in 18 (3.1%) and 10 (1.7%), respectively; and unplanned hospitalization leading to urgent revascularization in 15 (2.6%) and 11 (1.9%), respectively. Overall, the authors concluded that an FFR-guided strategy did not provide a significant benefit over an angiography-guided strategy with respect to the risk of death, myocardial infarction, or urgent revascularization at 1 year.
Finally, all patients enrolled in our study successfully underwent staged PCI and no significant cardiovascular adverse event was reported in between the culprit and subsequent hospitalization. Together with the above-described limited benefit in “hard outcomes” related to an early invasive treatment, we advocate that staged PCI might be considered in patients with “incidental” residual CAD (exclusive of left main stenosis and tight > 90% lesions) in case of the absence of morphological signs of vulnerability. Our current policy is to have a low threshold for PCI in any > 70% LAD stenosis as well as for FFR in case of 50–90% LAD stenosis. In case of non-LAD stenosis, the benefit of (FFR-guided) PCI should always be carefully weighted and justified, particularly in frail patients.
In conclusion, as atherosclerosis is a diffuse disease, a significant proportion of patients presenting with STEMI have residual stenoses in non-culprit territories currently defined as ≥ 50% stenosis in at least one major epicardial non-IRA. There are limited data supporting routine PCI of such lesions in order to reduce the rate of hard outcomes such as death or myocardial infarction. Moreover, it seems safe to postpone the completion of revascularization. While FFR provides a more “revascularization restrictive” approach, its benefit over visual estimation in the event of treatment of stable asymptomatic “incidental” stenoses still remains to be studied.