Chronic kidney disease (CKD), characterized by progressive damage and functional failure of the kidneys, is a major health problem worldwide due to its high prevalence and several complications, including atherosclerosis and cardiovascular disease [1–3]. When CKD reaches end-stage kidney disease stage, renal replacement therapy (i.e., hemodialysis (HD)) is instituted [1]. Atherosclerosis progresses and cardiovascular events occur due to the persistent oxidative burden, even under HD treatment [1, 4]; consequently, strategies to mitigate oxidative stress are warranted in the management of HD patients [4].
A high-density lipoprotein (HDL)-associated esterase called paraoxonase 1 (PON1), which is also known as arylesterase and paraoxonase (depending on the substrate employed in its measurement), inhibits the buildup of lipid peroxide on low-density lipoprotein, and low PON1 activity can raise the risk of cardiovascular events [5, 6]. In addition, PON1 has been proposed as an antioxidant biomarker for monitoring CKD [7].
Insufficient levels of vitamin C (ascorbic acid, a water-soluble antioxidant) have been linked to an increased risk of cardiovascular events in HD patients [8], and low blood levels of vitamin C have been found in critically ill patients who exhibit the oxidative burden [9]. Studies on vitamin C supplementation are thus ongoing as a potential treatment for the prevention of atherosclerosis and cardiovascular diseases as well as the improvement in oxidative burden [9–12]. To date, research has not established a relationship between vitamin C and PON1 activity in HD patients. Therefore, the objective of the present study was to report the pilot data on the effects of vitamin C supplementation on PON1 function in HD patients.
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) [13] reporting standards were followed to conduct the current review. The search was conducted using MEDLINE via PubMed, Embase via Dialog, and CENTRAL via Cochrane Library to identify original articles published before June 2023 on PON1 activity in HD patients with and without vitamin C supplementation. The keywords were combined with aryldialkylphosphatase, arylesterase, paraoxonase, vitami*, and ascorb*. From each study, the following data were extracted from the text or figures: authors’ names, year of publication, country, number of patients, method and duration of HD, vitamin C supplementation, and outcomes. We performed a random-effects model meta-analysis using the standard mean difference (SMD) with a 95% confidence interval (CI) between patients who received vitamin C and those who did not. Given the limited numbers of studies, a synthesized analysis of both paraoxonase and arylesterase activities was performed. The threshold for significance was p < 0.05.
Based on the methods, 422 articles were first identified using the keywords. After screening, four studies were finally eligible for the review (Table I) [14–17]. Two were pre- and post-intervention studies [14, 16], and two were case-control studies [15, 17]. Overall, there were 92 patients with a mean age of 39–66 years who were on HD for 4–5 h, 3 times a week, for a median of 40–80 months after induction of HD. The intervention consisted of the oral or intravenous vitamin C supplementation of 100–500 mg, daily or immediately after HD.
Table I
Summary of the included articles on PON1 activity in patients with and without vitamin C supplementation
| Author (country) [ref no.] | Year | Patient number (age) | Hemodialysis methods and durations | Vitamin C supplementation | PON1 without (before) vitamin C | PON1 with (after) vitamin C | Notes (additional findings of markers) |
|---|---|---|---|---|---|---|---|
| Hsu (Taiwan) [14] | 2007 | 10 (60 years) | 3 times/week for 4 h (polysulfone dialyzer), 40 months | 500 mg PO, immediately (once) at the end of dialysis session | 28 ±5 (μmol/ml/min; arylesterase) | 37 ±6 | RH2O2 reduced RHOCl unchanged |
| Ferretti (Italy) [15] | 2008 | 33 (66 years) | 3 times/week for 4 h, 80 months | 500 mg IV; at the end of each dialysis session for 6 months | 476 ±52 (U/ml; paraoxonase) | 769 ±121 | Lipid hydroperoxides reduced |
| Moradi (United States) [16] | 2010 | 20 (53 years) | 3 times/week (cellulose triacetate dialyzer), 33 months | 250 mg PO (with other antioxidants); once a day for 2 months | 95 ±8 (Kμ/l; arylesterase) | 100 ±9 | GPX unchanged |
| Sarandol (Turkey) a) vitamin C 500 mg, and b) 100 mg [17] | 2020 | 29 (39 years) | 3 times/week for 4–5 h, 66 months | a) 500 or b) 100 mg IV; at the end of each dialysis session for 4 months | 143 ±63 (U/l; paraoxonase) and 68 ±20 (kU/l; arylesterase) | a)177 ±70 (paraoxonase) and 63 ±20 (arylesterase) b) 168 ±77 (paraoxonase) and 72 ±19 (arylesterase) |
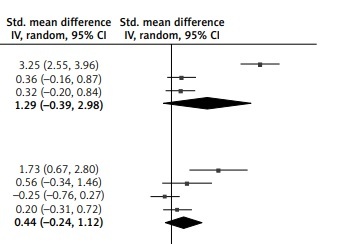
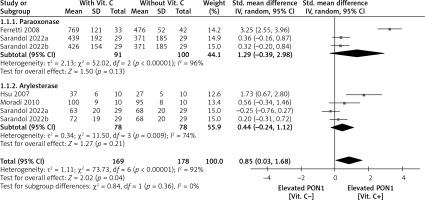
In the meta-analysis (Figure 1), there was no difference in paraoxonase activity between patients with (after) and without (before) vitamin C (SMD = 1.29; 95% CI: –0.39 to 2.98; p = 0.13) or arylesterase activity between them (SMD = 0.44; 95% CI: –0.24 to 1.12; p = 0.21). Finally, in the synthesized analysis, patients with vitamin C had significantly higher PON1 activity than those without (SMD = 0.85; 95% CI: 0.03–1.58; p = 0.04).
The present synthesized meta-analysis provided current pilot evidence that vitamin C supplementation might boost PON1 activity in HD patients. It may serve as a proof-of-principle that justifies further research into the relationship between vitamin C and cardiovascular pathologies related to PON1 in HD patients, as vitamin C-induced antioxidant activity recovery is an attractive scenario.
In HD patients, several substances (i.e., uremic toxins, inflammatory cytokines) are responsible for the PON1 dysfunction [18]. PON1 function may be compromised by oxidative stress, nitrative stress, and posttranslational modifications such as carbamylation, which reduce its activity or cause it to become dysfunctional [18]. On the other hand, vitamin C quenches hypochlorous acid (a proinflammatory oxidant) and mediates the loss of PON1 activity [19]. Additional biomarkers related to oxidative stress were measured in some studies that were included in the present meta-analysis (Table I) [14–17], and a reduction in lipid hydroperoxides was also reported to be correlated with an increase in PON1 [15]. Vitamin C helps quench lipid hydroperoxides by acting as a hydrosoluble recycler of oxidized tocopherol, which directly stops the chain oxidation of conjugated double bonds in polyunsaturated fatty acids [20]. These may partly explain the results of the present study.
The present analysis has some limitations. First, only a small number of studies met the criteria for inclusion in the meta-analysis, and they were single-arm studies. The cardiovascular outcomes were not examined. It is important to design large-scale, long-term cohort studies or randomized-controlled trials with relevant outcomes. Next, the quantities and modes of vitamin C delivery were not always unified across studies. Some investigators suggest an effective dose of intravenous vitamin C of 2 g/day [9] and an oral one of 0.5–1 g/day [11, 12], while as compared with the suggestions, the studies included in the present meta-analysis appeared to use comparatively low doses. Moreover, patients’ profiles might be considered. Vitamin C is typically safe in general populations, while it can even be helpful in certain populations (e.g., diabetes at an increased risk of cardiovascular diseases) [9, 12]. These points should be addressed in future.
In conclusion, based on the current pilot data using the meta-analysis, increasing PON1 activity by vitamin C supplementation seems to be possible in HD patients. For this claim to be supported, more research is required.