Introduction
In the United States there is a reported incidence of almost 800,000 acute coronary syndrome (ACS) events annually, and approximately 60% are non-ST elevation myocardial infarctions (NSTEMI) [1]. The electrocardiogram (ECG) is most often the first diagnostic test performed in suspected ACS and is given a class 1 indication in the clinical practice guidelines [2]. ST-segment elevation seen on ECG accurately localises the culprit vessel in ST elevation myocardial infarctions (STEMI) [3]. The utility of the ECG changes in localising coronary culprit territory in the setting of NSTEMI; however, it is not well established, and current literature is sparse. This study was conducted to evaluate the accuracy of the ECG distribution of non-ST elevation ischaemic changes and coronary culprit vessel identified by coronary angiogram in patients presenting with a percutaneous coronary intervention (PCI)-treated NSTEMI.
Material and methods
Study description and data source
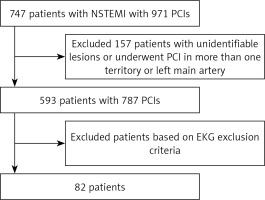
This was a single-centre retrospective study. The medical records of consecutive patients with NSTEMI, who underwent PCI between October 1, 2011 and November 30, 2017 were reviewed. ECGs for these PCI-treated NSTEMIs were reviewed in the ECG electronic medical record system “EKG MANAGEMENT” software (Figure 1).
Inclusion criteria included age ≥ 18 years, diagnosis of NSTEMI treated with PCI, presence of at least two ECGs in the electronic medical records, with at least one performed before and one after the PCI, and presence of dynamic ECG changes. Exclusion criteria included unidentifiable culprit lesion on the coronary angiogram, PCI in a left main coronary artery or more than one coronary artery, ECGs showing ventricular paced rhythm, left bundle branch block, fixed ST depression (STD) or T wave inversion (TWI) confined to right precordial leads with RsR’ in the presence of a right bundle branch block, fixed STD in the setting of left ventricular hypertrophy, and ST elevation meeting criteria for STEMI.
ECGs were performed using Mortara ELI 250 and ELI 280 electrocardiographs with a gain setting of 10 mm/mV, a paper speed of 25 mm/s, a high pass filter of 0.05 Hz, a lowpass filter of 150 Hz, and a notch filter of 60 Hz.
For patients who met the inclusion criteria, all ECGs during indexed hospitalisation were reviewed by two of the investigators (RG and TE). Differences relating to the presence or absence of diagnostic criteria were resolved by consensus. Deviation of ST-segment and amplitude of inverted T waves were documented for each lead separately.
Definitions
NSTEMI was defined as detection of a rise and/or fall of troponin values with at least one value above the 99th percentile above normal range and with at least one of the following: angina or angina equivalent, new ischaemic ECG changes (i.e. T wave inversion (TWI) and/or ST depression (STD)), or imaging evidence of new loss of viable myocardium and/or new regional wall motion abnormality in a pattern consistent with an ischemic aetiology.
Dynamic changes were defined as ischaemic ECG findings that were present in at least one lead on one or more ECG/s before and at least partially improved after PCI. These ischaemic changes included horizontal or down-sloping ST-segment depression ≥ 0.5 mm and/or T wave inversion ≥ 1 mm in two contiguous leads with a prominent R wave and R/S ration >1 [4].
ECGs with dynamic ischaemia were assigned to three locations (left anterior descending (LAD), right coronary artery (RCA), and left circumflex (LCX)) based on the seven different categories using previous recommendations for Q wave MI by the International Society for Holter and Noninvasive Electrocardiography, and then compared to a culprit coronary lesion in the coronary angiogram (Table I) [3].
Table I
Electrocardiographic (ECG) criteria used for localisation and nomenclature of non-ST elevation myocardial infarction (NSTEMI)
| Involved artery | Name of myocardial infarction | Involved ECG leads |
|---|---|---|
| LAD | Anterior or septal | [V1–V2] = sSeptal or mid-distal LAD [aVL ± I ± V2–V3] = First diagonal [V1–V2] with extension to [V3 ± V4–V6] without aVL or I = Mid LAD [V1–V6 + aVL ± I] = proximal LAD |
| Non-dominant* LCX or OM | Lateral | Q-wave equivalents of abnormally prominent R waves in leads V1–V2 lead I, aVL, and/or V5 and V6 |
| Dominant* RCA or LCX | Inferior | [II, III, aVF] |
Results
The initial search identified 747 patients, and the final cohort included 82 PCI-treated NSTEMIs of which 51% were males; 43.9%, 24.4%, and 31.7% received PCI to LAD, RCA, and LCX, respectively. In this cohort the sensitivity of ECG in localising single-culprit-vessel NSTEMI was 41.5%. The overall accuracy of ECG changes was 50.0%, 72.0%, and 70.0% in LAD, RCA, and LCX distribution, respectively. The sensitivity and specificity were 72.2% and 32.6% in LAD distribution, 20% and 88.7% in RCA distribution, and 15.4%and 82.1% in LCX distribution, respectively (Tables II and III). Of note, there were five NSTEMIs that received PCI to Ramus intermedius (which were not included in the analysis), four of them had LCX, and one RCA distribution ECG changes.
Table II
Pivot table showing detailed distribution of electrocardiographic (ECG) changes in percutaneous coronary intervention (PCI)-treated culprit vessel
| PCI location | ECG changes distribution | Total | ||
|---|---|---|---|---|
| LAD | RCA | LCX | ||
| LAD | 26 | 4 | 6 | 36 |
| RCA | 12 | 4 | 4 | 20 |
| LCX | 19 | 3 | 4 | 26 |
| Total | 57 | 11 | 14 | 82 |
Table III
Sensitivity, specificity, positive and negative predictive values, and accuracy of electrocardiographic changes distribution in localising percutaneous coronary intervention (PCI)-treated culprit vessel
| Test/ECG changes distribution | LAD | RCA | LCX |
|---|---|---|---|
| Sensitivity | 72.2% | 20.0% | 15.4% |
| Specificity | 32.6% | 88.7% | 82.1% |
| Positive predictive value | 45.6% | 36.4% | 28.6% |
| Negative predictive value | 60.0% | 77.5% | 67.6% |
| Accuracy | 50.0% | 72.0% | 70.0% |
Discussion
The distribution of surface ECG ischaemic changes (i.e. TWI and/or STD) were more sensitive in localising the culprit vessel of PCI-treated NSTEMI patients in LAD distribution but more specific in RCA and LCX distributions; the overall accuracy was modest. This is consistent with current literature, which reported that LAD coronary artery stenosis is correctly identified by ECG more often than the posterolateral circulation [5].
Ischaemia can cause no to several ECG changes, in isolation or combination, depending on multiple interrelated factors. The involved territory and artery, location within the culprit vessel (proximal versus distal), and transmurality can all contribute to the wide range of ECG findings [6, 7].
Generally speaking, incomplete coronary artery occlusion causes subendocardial ischaemia that may manifest as STD and/or TWI (non-ST elevation), while complete occlusion causes transmural ischaemia that is more likely to manifest as ST elevation [6, 7]. This, however, is not an absolute confederation. Up to 50% of NSTEMIs lack any evidence of ischaemic ECG changes, and approximately one-third of NSTEMIs have been shown to demonstrate complete occlusion of the culprit vessel with worse outcomes [8, 9]. Similarly, around one-third of STEMIs were found to have incomplete occlusion of the culprit artery and demonstrate better outcomes [9, 10]. In this study, of 593 patients identified as having NSTEMI, who underwent PCI, only 82 patients met the inclusion criteria, which further emphasises the wide spectrum of ACS and ECG accuracy in the localisation of culprit vessels.
The reported accuracy of ECG in localising STEMI is 83.1% and the sensitivity is 68.7% [11]. In this study, the sensitivity of ECG in localising PCI-treated NSTEMI was 41.5%. One such explanation for the higher false negatives is that NSTEMI patients, when compared to STEMI patients, are generally older with complicating comorbidities and increased likelihood of multi-vessel disease [12]. Additionally, the depth of subendocardial ischemia is variable and thus affects the magnitude and extent of ionic current disruption; therefore, the likelihood of the presence and type of surface ECG changes might differ depending on the extent of damage involved [6]. Cardiac magnetic resonance studies have shown that NSTEMI myocardial tissue injury is generally smaller than the STEMI infarct size, which makes it less likely to be reflected on the surface ECG [10]. Moreover, an impactful study showed that LAD infarcts affect on average about 40% of the ventricular myocardium, whereas RCA and LCX infarcts affect only 18% and 20%, respectively [13], which could explain the higher sensitivity of ECG changes in LAD distribution.
The ECG has been shown to be less sensitive in detecting posterior and lateral wall infarcts in NSTEMI as well as STEMI, especially when LCX is the culprit. The smaller size and/or more distal involvement of the LCX as a culprit vessel is more likely to lack transmurality, and the location of LCX territory opposite to the normal surface ECG leads may contribute to inconsistent ECG changes. Our study re-demonstrated the insensitivity of non-LAD ECG changes in the identification of culprit vessels in NSTEMI settings [14, 15]. The sensitivity of the surface ECG in non-LAD lesions could be affected by coronary dominance; however, the sensitivity remained poor despite taking dominance into consideration.
This study used strict inclusion and exclusion criteria to limit confounding variables. It is the largest study to date, to our knowledge, which evaluates the correlation between PCI-treated NSTEMI ECG changes and coronary territory involvement confirmed by coronary angiogram. The findings in the study outline that non-LAD ECG changes have poor sensitivity to localise the culprit vessel in NSTEMI. Other modalities, such as echocardiogram, to evaluate cardiac wall motion, and fractional flow reserve may further help to localise the culprit vessel in NSTEMIs where the multi-vessel disease with seemingly equivocal lesions can be seen in up to 59% of cases [16].
This is a relatively small single-centre retrospective study that limited further analyses such as location of the lesion (proximal versus distal) and size of the vessel on the surface ECG changes. It only included PCI-treated NSTEMIs and excluded medical therapy-treated NSTEMIs. It could be difficult, in some patients, to differentiate between type 1 and type 2 myocardial infarctions; it is unclear whether the type of myocardial infarction could affect the accuracy and sensitivity ECG changes.
In conclusion, this study shows that ischaemic non-ST elevation ECG changes had modest accuracy in localising culprit vessel in patients with PCI-treated NSTEMI. These changes were more sensitive in LAD distribution but more specific in RCA and LCX distributions. Larger studies are needed to confirm these findings.