Introduction
Chronic kidney disease (CKD) affects > 10% of the general population worldwide, amounting to > 800 million individuals [1]. Patients with CKD exhibit an elevated cardiovascular risk manifesting as coronary artery disease, heart failure, arrhythmias, and sudden cardiac death all resulting in more than half of deaths in this patient population [2].
Although traditional risk factors such as diabetes mellitus, dyslipidemia and hypertension very often coexist with CKD, additional nontraditional, uremia-related risk factors, such as inflammation, oxidative stress, vascular calcifications, platelet abnormalities and abnormal calcium-phosphorus metabolism are also implicated in the pathogenesis of cardiovascular disease (CVD) in these patients [3]. Both structural and functional alterations of the cardiovascular system are very common even in the early stages in CKD patients compared to the general population [4]. The incidence of heart failure and the subsequent cardiovascular mortality and morbidity increases as the estimated glomerular filtration rate (eGFR) declines, imposing the value of the early detection of the subclinical cardiac function deterioration [2, 4]. Myocardial function in end-stage renal disease (ESRD) patients has been studied extensively by conventional echocardiography, which provides merely semi-quantitative evaluation and cannot detect possible subclinical dysfunction. Traditional echocardiography indexes such as ejection fraction (EF) are not accurately sensitive tools enough to identify early myocardial dysfunction in this category of patients [5]. Moreover, mounting evidence supports that accelerated atheromatosis and ventricular remodeling lead to diastolic dysfunction whereas the systolic function may remain preserved.
Left ventricular global peak systolic longitudinal strain (LV GLS), obtained by two-dimensional speckle-tracking echocardiography (STE) with strain analysis, is a new more sensitive and objective assay for estimating cardiac functions among CKD patients [5–10]. Furthermore, it has a prognostic value. Krishnasamy et al. found that impaired LV GLS (> –16%) was associated with a 5.6-fold increase of the unadjusted risk of cardiovascular mortality in patients with advanced CKD and preserved EF [7]. LV GLS reflects the longitudinal contraction of the myocardium and its accuracy has been validated against tagged magnetic resonance imaging (MRI) [8]. It is derived from speckle tracking and is analyzed by post-processing apical images of the LV [8]. So the STE has emerged as a quotidian tool to detect subtle deformational abnormalities of systolic function which precede reduction of the left ventricular ejection fraction (LVEF) and can be used as a predictor of adverse events [9]. Frame-to-frame speckles are tracked and segmental strain is measured in an angle-independent manner [10].
Venous oxygen saturation is determined by the cardiac output and provides an estimation of body oxygen consumption/delivery ratio [11]. It declines during hemodialysis due to the reduction of the preload, the decrease of the arterial pressure, the myocardial stunning and the intermittent arrhythmias [12]. So, the venous oxygen saturation after the hemodialysis session would be used as a marker of myocardial dysfunction. Previous studies have shown that low oxygen saturation of the central vein blood (ScvO2) in conjugation with high ultrafiltration rates is associated with high mortality rates in HD patients [13, 14].
Moreover, hemodialysis can cause ischemic insult to various organs mainly via the ultrafiltration (UF)-induced reduction of the circulatory volume and the subsequent intradialytic hypotension (IDH) which may lead to perfusion mismatch [15]. Apart from the ultrafiltration rate, IDH can be also caused by myocardial stunning arrhythmias, valvular diseases as well as autonomic dysfunction [15–17]. So, it is difficult to identify subclinical ischemia caused solely by the UF.
Hemodialysis per se is a deteriorating factor that aggravates myocardial stress and injury in these patients [18–20]. Notably, hemodynamic abnormalities are frequent during hemodialysis and have been associated with increased mortality in these patients [20]. Echocardiographic studies before and after hemodialysis have shown that left ventricle EF as well as diastolic indices such as the early diastolic mitral annulus velocity (e’) are lower in patients who have intradialytic hypotension [21]. Similarly, LV GLS is also significantly lower in HD patients with mild to severe intradialytic hypotension [21, 22]. However, data regarding the function and performance of the right ventricle during hemodialysis are missing.
Despite significant advances in dialysis, the high mortality of ESRD patients is an important and unresolved issue. Identifying the high-risk patients could allow the physicians to optimize therapeutic interventions, which may lower morbidity and mortality [1, 2, 18, 23].
The goal of this prospective study is to investigate the alterations of myocardial strain as indicated by GLS of both left and right ventricles during hemodialysis in CKD patients undergoing hemodialysis. Furthermore, we aimed to test the hypothesis that the intradialytic variability of ScvO2 may be correlated with the GLS changes prior and post hemodialysis. In that case the degree of the ScvO2 decrease may reveal a subclinical ischemic insult that may occur during the hemodialysis session and identify the group of the high cardiovascular risk patients that could benefit from the secondary prevention therapy.
Material and methods
This is a prospective study conducted between January 2022 and December 2022 in HD patients of the General Hospital of Chania. We enrolled 37 patients who were undergoing HD via a permanent or temporal central venous catheter. All patients were maintained in regular HD 3 times per week, of 4 h each session. We excluded patients with known severe heart failure defined as EF < 40%, severe valvular disease, asthma and chronic obstructive pulmonary disease as well as those with a history of malignancy, chemotherapy or radiotherapy, thyroid disease and any severe infectious diseases during the last 6 months. The demographic as well as other data of the patients are summarized in Table I. A blood sample from the central vein was obtained before and after hemodialysis in order to calculate ScvO2 by blood gas analysis. High sensitive troponin measurements were obtained in the patients before and after the HD session. Clinical examination and complete echocardiographic evaluation were performed. Blood pressure (BP) and heart rate (HR) were monitored during HD. Written informed consent was obtained from all patients prior to their enrolment in the study. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution’s human research committee.
Table I
Patient demographic data
| Parameter | Results |
|---|---|
| Number (n) | 37 |
| Males/females (n) | 20/17 |
| Age [years] | 62.1 ±15.1 |
| Time on HD, range [months] | 32.4 ±41.8 13–87 |
| Diabetic | 35.1 (%) |
| Hypertensive | 70.3 (%) |
| Smokers (%) | 42.1 (%) |
| CAD (%) | 35.1 (%) |
Echocardiographic evaluation
A standard M-mode and 2-dimensional (2D) echocardiographic study was performed in all participants, according to the recommendations of the American Society of Echocardiography (ASE) [24], before and after HD. Pulsed-wave and continuous-wave Doppler tracings were obtained and ventricular volumes and LVEF were calculated by the modified Simpson method using apical 4- and 2-chamber views. STE was also performed according to the ASE recommendations [24]. The 2D speckle-tracking strain analyses were performed on grayscale images of the left ventricle, using Echopac instrument (Version BT13, GE Medical Systems), and peak GLS was measured. Loops of 3 cardiac cycles were stored digitally and analyzed offline. The optimal images for speckle tracking were selected. If one or more segments were not clearly visible, the image was excluded. For each platform, a region of interest (ROI) was placed by user-defined markers to incorporate the entire myocardial wall. Repeat adjustments of the ROI were done if tracking quality was insufficient. The frame rate for speckle-tracking strain assessment was 50–90 frames/second. During strain analysis, the endocardial border was manually traced at end-systole and the width of the region of interest was manually adjusted to include the entire myocardial wall thickness. The Echopac software then automatically tracks and accepts segments with good tracking quality and rejects poorly tracked segments. However, the operator can manually override computer-generated tracking and accept or reject individual segments based on visual assessments of the tracking quality. Measurements were made throughout the cardiac cycle in the apical 4-chamber, 2-chamber, and long-axis views. LV GLS was obtained by averaging peak values of segmental strain in the apical views. For the assessment of the right ventricle GLS (RV GLS) we evaluated the peak global strain of the whole myocardium including the septum and the free wall in the apical four-chamber view focused on the right ventricle. The GLS value is expressed as a negative algebraic (%) number, so more negative values of GLS reflect better myocardial performance.
All echocardiographic assays were performed by the same investigator (SM).
Statistical analysis
The summary of descriptive statistics is presented as mean ± standard deviation (SD) for continuous categorical variables. The paired samples t-test or paired Wilcoxon test was used for assessing the effect of treatment, if the distribution was parametric or nonparametric, respectively. Multiple logistic regression analysis was applied to identify the correlations among several variables.
Results
Our study comprised 37 patients (54% male, 46% female) with a mean age of 62.1 ±15.1 years, 18 (70.3%) patients were hypertensive, 12 (32.4%) were diabetic, 13 (35.1%) with a known coronary artery disease and 16 (42.1%) were smokers. The mean duration in HD was 32.4 ±41.8 months (range: 13–87 months). The causes of ESRD were hypertensive nephropathy in 13, diabetic nephropathy in 14, polycystic kidney disease in 2, glomerulonephritis in 4, interstitial nephritis in 2, obstructive nephropathy in 1 and ESRD of unknown origin was present in 1 patient. The main characteristics of our patients are demonstrated in Table I.
All patients were dehydrated through ultrafiltration (UF) during the HD session. The mean UF rate (UFR) was 730 ±22 ml/min. Patient’s mean body weight before and after HD was 80.45 ±17.42 and 77.43 ±16.81 kg, respectively, p < 0.01. The mean ScvO2 before the hemodialysis session was 76.47 ±1.98 whereas the post hemodialysis it was 71.54 ±5.10%, p < 0.01.
The LV GLS mean value before hemodialysis was –17.73 ±3.44, whereas at the end of the HD session the GLS mean value increased to –14.21 ±3.44, p < 0.05. On the other hand, the RV GLS did not show differences before and after HD, –17.08 ±3.91 and –16.77 ±3.81, p = ns. The LVEF was normal when calculated by the Simpson method while there was no significant difference found in the values before and after HD, 47.43 ±13 vs. 49.39 ±11.89%, ns.
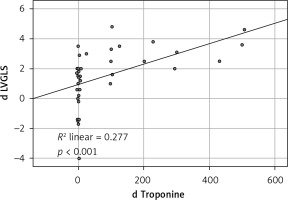
The mean HST levels before HD were 22.45 ±13.2 whereas post HD were 106.38 ±14.6 pg/ml, p < 0.01. All calculations before and after HD are shown in Table II.
Table II
Calculations before and after HD
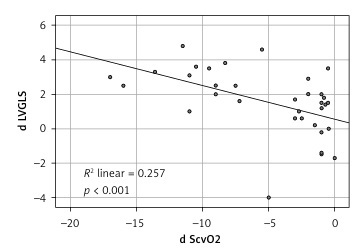
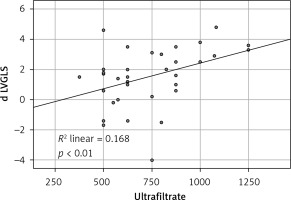
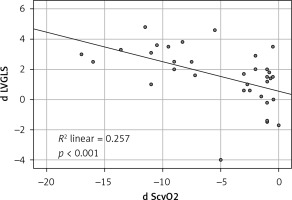
ScvO2 was significantly correlated with the UFR during hemodialysis (Pearson Correlation: r = 0.635, p < 0.001): the higher ultrafiltration rates caused greater reduction in ScvO2 levels. We also found an association between UF rate and post dialysis troponin levels: r = –0.568, p < 0.001 (Table III).
Table III
Univariate Spearman correlations of the differences of the calculations before and after HD
Like the ScvO2 there was an inverse correlation between the reduction in LV GLS and the ultrafiltration rate (Table III).
The increase of the algebraic value which means deterioration in the LV GLS mean value after the termination of the HD session was found to be correlated with the troponin level increase as well as with the reduction of ScvO2 levels, p ≤ 0.001 and p < 0.001, respectively. On the other hand, the RV GLS did not show any interdependence with ScvO2 as well as with the HST levels.
The multiple regression analysis showed that the reduction of ScvO2 was correlated with the alteration of the LV GLS, with the increase of the HST as well as with the UFR (Table IV).
Table IV
Multiple logistic regression analysis: correlation between the reduction of SAT O2 and several variables (only the statistical important)
| Predictor | dSAT O2 | Estimate | SE | t | 95% CI | P-value | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| d left ventricle GLS | 0.15 | 0.06 | 2.29 | 0.03 | 0.56 | 0.038 | |
| UFR | –0.06 | 0.03 | –2.26 | –0.65 | –0.03 | 0.042 | |
| Troponin | 0.01 | 0.00 | 2.56 | 0.07 | 0.59 | 0.025 | |
All single correlation relationships as well as the correlation diagrams are shown in Table III and in Figures 1–3.
Figure 1
Correlation between the differences of mean left verticle GLS before and after HD and the differences of central vein oxygen saturation before and after HD
Discussion
This is a prospective observational study which was the first to investigate the utility of ScvO2 levels in predicting subclinical myocardial dysfunction in HD patients assessed by echocardiographic deformation indexes and troponin values. We showed for the first time that a greater ScvO2 reduction after hemodialysis results in lower values of LV GLS as well as higher values of HST values indicating the presence of a subclinical myocardial injury.
It is well known that ESRD patients are of high risk for cardiovascular events, so the early detection of subtle LV systolic dysfunction is crucial for the prognosis of these patients [25]. Hemodialysis may cause subclinical myocardial ischemia due to hypotension as well as the decrease of the preload as a result of the ultrafiltration [15–17, 25]. A previous study evaluated the extent and severity of HD-induced cardiac injury using intradialytic 2D speckle-tracking strain echocardiography and found that 60% of patients developed HD-induced myocardial stunning, which was associated with the UFR and the intradialytic hypotension [20].
Furthermore, HD patients are characterized by fluid overload which results in LV hypertrophy, fibrosis and diastolic dysfunction as a consequence of LV remodeling [2, 3, 26, 27]. These altered myocardial mechanics cannot be detected well by conventional echocardiographic indexes which have failed to predict myocardial function deterioration [28, 29]. Ejection fraction, used as the standard method for measurement of the systolic function, lacks sensitivity in the detection of subclinical myocardial dysfunction both in general as well as in the hemodialysis patient population [30–32]. In the early stages of myocardial dysfunction, the most common abnormality observed is an impaired longitudinal myocardial contractility whereas in the later stages, both radial and circumferential functions are affected resulting in LVEF reduction [31]. Increasing data from the literature indicate that the 2D speckle-tracking strain analysis with 2D strain analysis may be an effective tool for the assessment of subclinical disturbances when LVEF is still normal [30–32].
LV GLS assessed by the 2D speckle-tracking strain analysis is the ratio of the maximal change in myocardial longitudinal length in systole to the original length and is capable to unmask subtle myocardial dysfunction [9, 33]. Furthermore, it has been shown that it also can predict cardiac adverse events [9, 33, 34]. Our study confirmed this issue. Although LV EF was found to be unaffected by the dialytic procedure as it was estimated by the conventional echocardiographic assay, the 2D strain analysis revealed a significant deterioration of the GLS at the end of hemodialysis which was associated directly with the ultrafiltration rate.
All these findings suggest that GLS is more sensitive than LVEF as a marker of LV dysfunction [35]. This fact results in the speculation that many patients classified as normal by LVEF would be abnormal by GLS, suggesting that there is heterogeneity of the intrinsic myocardial function in patients with normal LVEF [36].
ScvO2 may be a useful marker of ultrafiltration-driven hypoxia during HD [11–14]. The fall in ScvO2 is caused by an imbalance between O2 consumption/delivery ratio in the tissues as a result of the HD-induced hypoxia [12]. In acute coronary syndromes, venous blood oxygen saturation has been used to identify patients at risk of heart failure or cardiogenic shock [37]. In another study it has been demonstrated that in patients with advanced heart failure the ScvO2 is inversely correlated with adverse events [38]. Patients with ScvO2 > 60% had lower incidence of major adverse events, faster dobutamine weaning and finally had a better survival [38].
Though it remains uncertain if the ScvO2 can be used as a marker of quantification of the severity of tissue hypoperfusion, it seems to have a prognostic value. We observed a significant reduction of ScvO2 after hemodialysis in our patients which also was correlated with the degree of the ultrafiltration.
In the present study the speckle tracking 2D echocardiography revealed a subclinical LV dysfunction in patients with a higher rate of ScvO2 reduction as evaluated by LV GLS values. Thus, lower levels of O2 delivery as a result of higher UFR probably impaired myocardial deformation parameters. This ischemic insult was also demonstrated by higher levels of troponin measured at the end of the HD session.
Although the systolic function of the ESRD patients may remain intact, fibrosis deposition due to uremia-related factors remains an important cause of adverse events [39]. Interstitial fibrosis results in decreased capillary density, ischemia and increased risk of sudden cardiac death [39, 40]. GLS not only provides quantitative assessment of myocardial function but also reflects changes in the myocardial interstitium including the extent of myocardial fibrosis [30]. It is a more reliable prognostic marker of CV events and mortality in CKD patients than the conventional echocardiographic parameters [41]. In this study the multiple logistic regression analysis proved a significant correlation between the reduction of the ScvO2 and the reduction of LV GLS. The reduction of ScvO2 was found to be an independent prognostic marker of the worsening of LV GLS (p = 0.038).
Taking into account that higher UFR further aggravates ischemia and deteriorates myocardial systolic function, we may speculate that ScvO2 may therefore be used as a tool to identify those patients at greater risk of dialysis-induced myocardial ischemia. If we take into consideration that this marker is easily applicable, it may designate those patients that could benefit from the secondary prevention therapy. The findings of our study, i.e. the deterioration of both LV GLS and ScvO2 as well as the elevation of troponin, all of them indicating a cardiac stress during the dialysis session, may suggest that hemodialysis per se triggers a myocardial functional and tissue injury and probably is one of the missing links of the inappropriate high prevalence of CVD in the patients undergoing hemodialysis.
In conclusion, our prospective observational study demonstrated that the ScvO2 decline during HD is associated with a deterioration of LV GLS and an increase of troponin values, indicating functional or/and tissue myocardial injury. Both LV GLS and HST are powerful predictors for cardiovascular adverse events, so ScvO2, which is a quite easily applicable tool in our everyday practice, may predict adverse events in HD patients. However, LV GLS and HST are surrogates. Clinical studies including a greater number of patients could address the issue if the ScvO2 decline after the HD session is capable to predict HD patient’s outcome.
Our study has some limitations. First, this is a single-center study. Another limitation is the relatively small sample size.