Introduction
It is estimated that approximately 783.2 million people worldwide will be diagnosed with diabetes in the year 2045, while the projection for the year 2050 suggests that one in three individuals will experience some type of diabetes [1–4]. Approximately, 5% of all patients with diabetes are diagnosed with type 1 diabetes (T1D) and the rest 90–95% with type 2 diabetes (T2D). Interestingly, almost one in nine women have diabetes, while 35% of individuals with newly diagnosed diabetes are women of reproductive age, suggesting that diabetes is increasingly affecting women of childbearing age [5].
Pregestational diabetes is described when a woman with diabetes before the onset of pregnancy becomes pregnant and consequently she is vulnerable to higher risk for adverse outcomes in the embryo/foetus, including spontaneous abortions [5, 6]. Structural abnormalities (mainly cardiovascular, neurological and urogenital) have been closely associated with the severity of hyperglycaemia before conception and during organogenesis (5–8 weeks after the last menstrual period) [6, 7]. Uncontrolled glucose levels after organogenesis have been strongly associated with large-for-gestational-age babies, macrosomia, neonatal hypoglycaemia, shoulder dystocia, neonatal hyperbilirubinemia and respiratory morbidities [6, 8]. Long-term adverse effects for the child include higher risk of childhood obesity, metabolic syndrome, T2D and other risk factors for future cardiovascular diseases [6, 8].
Insulin therapy (both in multiple daily injections and continuous subcutaneous insulin infusion) is the cornerstone of pharmaceutical therapy in pregestational diabetes, to achieve tight/strict glycaemic control (ideally hemoglobulin A1c (A1c) levels < 6–6.5% during early gestation and < 6% during the second and third trimesters of pregnancy) [6, 9]. Strict glycaemic control, with minimal glucose variability, starting from before conception and maintained throughout pregnancy decreases significantly adverse foetal and maternal outcomes; maternal hypoglycaemic episodes are the major barrier in achieving this goal [6–9]. Short-acting insulin analogues (insulin aspart and insulin lispro) are preferred compared to human insulins, since they can mimic better the physiological secretion of insulin, they are more flexible and they exert lower risk for hypoglycaemia [6, 9, 10].
Long-actin insulin analogues are also preferred versus human intermediate-acting insulin (Neutral Protamine Hagedorn (NPH)) because they exert longer-acting mode of their effects without sharp peaks/falls, less fluctuations and less episodes of hypoglycaemia. Insulin detemir and insulin glargine were the main therapeutic options for the coverage of the basal insulin rhythm in women with pregestational diabetes for the last decade [10, 11]. Insulin detemir gained approval for the treatment of diabetes in pregnancy after the results of a large randomised controlled trial (RCT), which demonstrated lower fasting glucose levels with similar rates of hypoglycaemic episodes and hemoglobulin A1c values versus insulin NPH; its safety was also verified in a large multinational observational study [12, 13]. As far as insulin glargine (both U100 and U300) is concerned, there are currently no RCTs to investigate its use in pregnancy. Reassuring observational evidence and data from a large meta-analysis have not shown any safety issues and any significant efficacy differences versus insulin NPH [11, 14–16]. However, when they are administered once a day, glucose-lowering effects of both insulin detemir and insulin glargine tend to fluctuate considerably over 24 h [17]. Patients with T1D, who use low basal insulin doses at night, usually have to inject twice daily so as to avoid late afternoon hyperglycaemic spikes.
Insulin degludec
Insulin degludec (IDeg) is an ultralong-acting analogue, which has half-life of over 25 h and full duration of effect of more than 42 h, reaching a steady-state serum concentration after 2–3 days of its administration [18]. It promotes flat, steady, peakless and predictable insulin concentrations, with minor intra-individual and inter-individual variability (approximately one-fourth glycaemic variability versus insulin glargine U100) [18, 19]. Indeed, it was demonstrated that after one dose of IDeg, its glucose-lowering activity and total body exposure were more evenly distributed versus the other basal insulins in patients with both T1D and T2D [18]. IDeg can be administered once daily at any time of the day and has been approved for the treatment of diabetes mellitus in adults, adolescents and children of at least one year of age [18, 19]. Its cardiovascular safety profile, in a population of patients with T2D and high cardiovascular risk, was illustrated when it was compared to insulin glargine U100 [20].
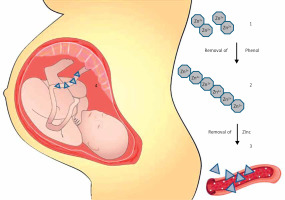
IDeg has a protein sequence based on human insulin, which was adjusted by acylating DesB30 at the e-amino group of LysB29 with hexadecandioic acid through a c-L-glutamic acid linker [21]. With the addition of phenol (a stabilizing excipient) IDeg forms soluble and stable di-hexamers in its pharmaceutical formulation, since only one of the ends of a hexamer can bind to the side chain of another IDeg hexamer. After its subcutaneous injection, phenol quickly diffuses away from the initial molecule and IDeg di-hexamers open at both ends. This reaction promotes the formation of a soluble depot of long and stable multi-hexamer chains [18, 19]. The hexamers are connected one to another by a single junction between one of the fatty-acid molecules and the zinc-containing core of an adjacent hexamer [22]. The slow and gradual diffusion of zinc from these multi-hexamers promotes gradual, stable and extended release of monomers to the circulation. The release of monomers to the circulation is considered the rate limiting step for IDeg absorption and can promote detectable serum levels even after 96 h from the time of its injection (Figure 1) [19, 21–23]. Contrastingly, insulin glargine forms microprecipitates after its subcutaneous injection, which have to be re-dissolved so as to be efficiently absorbed, rendering its absorption variable and relatively unstable [24].
Figure 1
Insulin degludec: mechanisms of activity and umbilical cord penetration. 1. IDeg has a protein sequence based on human insulin, which was adjusted by acylating DesB30 at the e-amino group of LysB29 with hexadecandioic acid through a c-L-glutamic acid linker. With the addition of phenol, insulin degludec (IDeg) forms soluble and stable di-hexamers in its pharmaceutical formulation, since only one of the ends of a hexamer can bind to the side chain of another IDeg hexamer. 2. After its subcutaneous injection, phenol quickly diffuses away from the initial molecule and IDeg di-hexamers open at both ends. This reaction promotes the formation of a soluble depot of long and stable multi-hexamer chains. 3. The slow and gradual diffusion of zinc from these multi-hexamers promotes gradual, stable and extended release of monomers to the circulation. The release of monomers to the circulation is considered the rate limiting step for IDeg absorption. 4. A recent exploratory post hoc analysis of the EXPECT study suggested that mean umbilical cord blood IDeg levels were 44 (range: 10–257 pmol/l). Mean cord blood IDeg levels were similar in the 18 neonates who experience hypoglycaemic episodes (41.3 pmol/l) vs. the remaining 47 neonates (45.1 pmol/l)
T1D – type 1 diabetes.
IDeg can bind reversibly to plasma albumin; this ability can mainly suppress any acute changes of its absorption rather than contributing to its prolonged activity [22, 24]. Of great importance is the fact that IDeg is a strong agonist of the insulin receptor and has minimal affinity for the receptor of insulin-like growth factor 1 (IGF-1, almost 2% of regular insulin). These properties establish a low mitogenic/metabolic potency ratio for this insulin, which has a special interest in case of possible gestation [22–24]. IDeg also has a minimal risk of immunogenicity, and no significant connection was shown between the development of cross-reacting antibodies with the total insulin dose, A1C levels and/or episodes of hypoglycaemia [24]. Indeed, the policy of several diabetes centres worldwide was to permit women who were treated with IDeg to continue this drug during their pregnancy after they were informed about the benefits and possible disadvantages of this treatment strategy. This review examines thoroughly all current evidence of IDeg in pregestational diabetes as well its future role in this population.
Preclinical data
Preclinical studies in rats and in rabbits during the time of embryo foetal development explored the possible effects of IDeg versus insulin NPH in fertility, embryo foetal evolution and pre/post-natal development. Visceral/skeletal abnormalities and pre/post implantation losses were reported when IDeg was administered subcutaneously at up to 21 U/kg every day in rats and 3.3 U/kg daily in rabbits, resulting in 5 and 10 times the subcutaneous dose of 0.75 U/kg/day, respectively [25]. The effects of IDeg were like those found for insulin NPH (possibly secondary due to maternal hypoglycaemia). However, the higher risk of embryotoxicity and other safety reproductive issues were not shown in animal reproduction studies after IDeg administration. IDeg was also isolated in the milk of lactating rats [25].
Clinical evidence
After a few years of the approval of IDeg in Europe and the USA, several case reports and case series were published and presented data of its use in pregnancy [26–31]. These reports included mainly women with T1D and suggested that all infants were liveborn without any congenital malformations. They reassuringly demonstrated no embryo-foetal toxicity and highlighted the urgent need for further studies, to establish the safety and efficacy of IDeg in pregnancy.
The first cohort study that explored the role of IDeg in pregnancy was published by Keller et al. in 2019 [32]. In this small observational study, data from 22 women with T1D treated with IDeg and 51 women on insulin glargine from conception to delivery were analysed retrospectively (the individual clinical data were available in 88–100% of the population enrolled). For patients with more than one pregnancy during the study period (approximately 4 years in the IDeg arm and 2.5 years in the glargine arm), only the latest pregnancy was analysed. Pregnant patients who delivered by planned caesarean section in the IDeg arm were advised to stop insulin therapy on the morning of the procedure, while those who delivered vaginally omitted the first IDeg dose on the morning of the delivery, aiming to decrease IDeg levels immediately and avoid severe post-delivery hypoglycaemic episodes. A1c levels were decreased similarly in both arms in late pregnancy. Severe hypoglycaemic episodes were also similar in both groups (p = 1.0) and none of the participants experienced severe hypoglycaemia after delivery during their hospitalization. No significant differences in any obstetrical or neonatal outcomes (including congenital malformations and perinatal death) were reported between the two arms. No safety issues by continuing IDeg during pregnancy were also shown. Most women in the IDeg group (96%) injected IDeg once a day, while 63% of women in the glargine group needed two or more injections every day in late pregnancy. The main limitations of this study were its retrospective origin and the limited number of pregnant women in the IDeg arm.
Another small retrospective observational study explored perinatal and obstetrical data from 12 women (27.5 ±8 years of age) with T1D (duration of 14.9 ±7.4 years), who became pregnant while using IDeg in the periconceptional period [33]. Their mean pre-pregnancy weight was 84.8 ±12 kg and the mean periconceptional A1c levels 7.8 ±1.5%. The dose of IDeg at the time of conception was 0.42 ±0.16 U/kg. IDeg was suspended at 9.5 ±4.8 weeks of pregnancy. Six women were treated with insulin glargine, five with insulin detemir and one patient with insulin NPH. From the six births (all of them by caesarean section) mean newborn weight was 3438 ±690 g. Neonatal hypoglycaemia was described in three births, while none of the infants presented with respiratory distress, jaundice, congenital malformations or experienced admission to the neonatal intensive care unit.
Interesting results were reported from a secondary analysis of a prospective observational cohort of pregnant women with T1D or T2D that focused on preeclampsia [34]. A total of 162 consecutive singleton women were included in the final analysis; 15 of the women using IDeg were included in the previous publication of the same scientific group [32]. The participants using IDeg generally started this insulin as a part of their routine treatment, before and unrelated to the current pregnancy. They achieved satisfactory glycaemic control and they decided to continue IDeg during pregnancy after they were informed about the benefits and possible disadvantages. Eventually 67 women were treated with IDeg and 95 used other long-acting insulin analogues, namely: (i) insulin glargine U100 (n = 58); (ii) biosimilar insulin glargine U100 (n = 2); insulin glargine U300 (n = 9); (iv) insulin detemir (n = 24) and (v) insulin NPH (n = 2). No significant differences in all maternal outcomes, including preterm delivery (16% vs. 27%) and preeclampsia (10% vs. 8%), were described between the IDeg group and the other basal insulin groups, respectively. All neonatal outcomes were similar between the two arms and no perinatal deaths were found. In general, no clinical and safety concerns were described by continuing IDeg during pregnancy in this population under real-world conditions. The observational origin of the study, confounding by indication and lack of data for basal insulin doses and episodes for severe hypoglycaemia were the main limitations of this study.
EXPECT was an open-label, parallel-group, multinational, treat-to-target, active-controlled, non-inferiority study, which explored the safety of IDeg versus insulin detemir in 225 pregnant women of at least 18 years of age with T1D for at least one year, who were experiencing 8–13 weeks of gestation (they received a trial drug from randomization) or planned to become pregnant within 52 weeks of randomization (they received a trial drug before conception and during pregnancy until 28 days post-delivery or for 12 months if pregnancy was not achieved) [35]. All women were randomised to receive subcutaneously either IDeg once daily (n = 111, 92 were pregnant during the trial) versus detemir once or twice daily (n = 114, 96 were pregnant during the enrolment), both with mealtime insulin aspart 2–4 times every day. Eventually, 178 (95%) of 188 women who were pregnant during the trial completed the study period. At screening most women (42%) were treated with insulin glargine (U100 or U300).
The last planned A1c levels before delivery in the IDeg arm were 6.2% versus 6.3% in the detemir arm, confirming non-inferiority for IDeg vs. detemir. The percentage of women who experience last planned A1c of no more than 6.5% before delivery was 69% in the IDeg arm vs. 63% in the detemir group (p = 0.062). At 36 gestational weeks, the mean daily basal insulin dose in the IDeg arm was 33.2 units vs. 38.3 units in the detemir group, while the mean daily bolus dose was similar between the two treatment arms. No significant differences were described for rates of any hypoglycaemia (p = 0.82) and nocturnal hypoglycaemia (p = 0.59) during the pregnancy period between the two groups. Mean change in body weight before delivery was 12 kg vs. 10.8 kg in the IDeg and detemir groups, respectively. No clinically relevant differences in maternal adverse outcomes and other serious safety events were observed between the treatment arms. Potential trends for an increased risk of preterm delivery (34% vs. 22% of live births) and preeclampsia (14% vs. 8%) in the IDeg arm versus the other arm could be attributed to: (i) the numerically higher maternal bodyweight women receiving IDeg; (ii) the higher numbers of nulliparous women and (iii) switching to IDeg from other basal insulins early in pregnancy in women who were already pregnant vs. the detemir arm. However, in the previous studies that were described, no trends for these parameters were found [32, 34].
In terms of foetal and infant outcomes, there were no significant perinatal or neonatal deaths, and evidence was generally similar between the two arms for major congenital abnormalities and early foetal loss. The mean birthweight of infants was 3691 g in the IDeg arm versus 3490 g in the detemir group. A potential trend for an increased risk of infants born large for gestational age was reported in the IDeg arm (64% vs. 51% of live births) in contrast to previous studies [32, 34]. The percentage of infants, who experience adverse events after delivery and until the final follow-up visit was 63% in the IDeg arm compared to 67% in the detemir arm. The proportion of infants with neonatal hypoglycaemia during the first 24 h after birth was 23% and 22% in IDeg and detemir groups, respectively. Interestingly, a recent exploratory post hoc analysis of the EXPECT study investigated the possible correlation between neonatal hypoglycaemia and umbilical cord blood levels of IDeg in 66/86 neonates of women in the IDeg arm [36]. Mean umbilical cord blood IDeg levels were 44 (range: 10–257 pmol/l). Mean umbilical cord blood IDeg levels were similar in the 18 neonates who experience hypoglycaemic episodes (41.3 pmol/l) and the remaining 47 neonates (45.1 pmol/l). Among neonates with hypoglycaemia (plasma glucose levels ≤ 30 mg/dl), no specific correlation was found between neonatal plasma glucose levels and IDeg umbilical cord blood levels providing reassurance regarding the safety of this insulin in pregnant women with T1D. Conclusively, the findings of the EXPECT study were reassuring for the use of IDeg in diabetic women who are pregnant or are planning to become pregnant, since detemir has been well studied and is considered effective and safe in this setting. Its administration has been recently approved for pregestational diabetes in the USA, Canada and Europe [37, 38]. The main results of current clinical studies, which explored the role of IDeg in pregestational diabetes, are shown in Table I.
Table I
Clinical studies of IDeg in pregestational diabetes: main results
| Ref./year* | Study population | Study design | Major results |
|---|---|---|---|
| Keller et al. [32]/2019 | 22 women with T1D treated with IDeg and 51 women on insulin GLAR. | Small observational study. Data from 22 women with T1D treated with IDeg and 51 women on insulin GLAR from conception to delivery were analysed. For patients with more than one pregnancy during the study period (approximately 4 years in the IDeg arm and 2.5 years in the GLAR arm) only the latest pregnancy was analysed. | No significant differences in any obstetrical or neonatal outcomes (including congenital malformations and perinatal death) were reported between the two arms. No safety issues by continuing IDeg during pregnancy were also found. |
| Martínez-Montoro et al. [33]/2019 | 12 women with T1D. | Small retrospective observational study, which explored perinatal and obstetrical data from 12 women (27.5 ±8 years of age) with T1D (duration of 14.9 ±7.4 years), who became pregnant while using IDeg in the periconceptional period. IDeg was suspended at 9.5 ±4.8 weeks of pregnancy. | From the six births (all of them by caesarean section), mean newborn weight was 3438 ±690 g. Neonatal hypoglycaemia was described in three births, while none of the infants presented with respiratory distress, jaundice, congenital malformations or experienced admission to the neonatal intensive care unit. |
| Ringholm et al. [34]/2022 | 162 consecutive singleton women with T1D or T2D. | Secondary analysis of a prospective observational cohort of diabetic pregnant women focused on preeclampsia. Eventually 67 women were treated with IDeg and 95 used other long-acting insulin analogues: (i) insulin GLAR U100 (n = 58); (ii) biosimilar insulin GLAR U100 (n = 2); insulin GLAR U300 (n = 9); (iv) insulin DET (n = 24) and (v) insulin NPH (n = 2). | No significant differences in all maternal outcomes, including preterm delivery (16% vs. 27%) and preeclampsia (10% vs. 8%), were described between the IDeg group versus the other basal insulin groups respectively. All neonatal outcomes were similar between the two arms and no perinatal deaths were found. |
| Mathiesen et al. [35]/2023 | 225 pregnant women with T1D for at least 1 year. | EXPECT was an open-label, parallel-group, multinational, treat-to-target, active-controlled, non-inferiority study. All participants were randomised to receive either IDeg once daily (n = 111, 92 were pregnant during the trial) versus DET once or twice daily (n = 114, 96 were pregnant during the enrolment), both with mealtime insulin aspart 2–4 times every day. | No significant differences were described for the rates of any hypoglycaemia (p = 0.82) and nocturnal hypoglycaemia (p = 0.59) during the pregnancy period between the two groups. No clinically relevant differences in maternal adverse outcomes and other serious safety events were observed between the treatment arms. In terms of foetal and infant outcomes, there were no significant perinatal or neonatal deaths, and evidence was generally similar between the arms for major congenital abnormalities and early foetal loss. |
| Mathiesen et al. [36]/2023 | 66/86 neonates of women who were treated with IDeg. | Post hoc analysis of the EXPECT study. It explored the possible correlation between neonatal hypoglycaemia and umbilical cord blood levels of IDeg. | Mean cord blood IDeg levels were similar in the 18 neonates who experience hypoglycaemic episodes (41.3 pmol/l) and the remaining 47 neonates (45.1 pmol/l). Among neonates with hypoglycaemia (plasma glucose levels ≤ 30 mg/dl), no specific correlation was found between neonatal plasma glucose levels and IDeg umbilical cord blood levels. |
Conclusions and perspectives
The unique pharmacokinetic and pharmacodynamic characteristics of IDeg as well as its low mitogenic/metabolic potency ratio establish this insulin as an excellent option for women with pregestational diabetes [17, 20–22]. Moreover, substantial evidence has shown that switching to IDeg from different basal insulins (both in patients with T1D and T2D) was cost effective and was associated with improved glycaemic control, higher time in range, less glycaemic variability in difficult to control populations, less basal and total insulin doses and lower risk of hypoglycaemia (overall and nocturnal), making IDeg a valuable tool in our therapeutic armamentarium in pregestational diabetes [39–44]. However, in women with pregestational diabetes who experience hyperglycaemic spikes during the early morning hours (mainly patients with T1D) and/or experience basal rate profiles with significant intraday variability as pregnancy progresses, IDeg may not be the proper therapeutic approach due to its flat and stable blood glucose-lowering effect; continuous subcutaneous insulin infusion should be the preferred therapeutic approach in this setting [45–47].
Results from future well-designed observational studies enrolling larger populations of pregnant women with pregestational diabetes will be required to provide further evidence for any possible relatively uncommon safety outcomes with low incidence rates. Individualizing and adjusting basal insulin to women with pregestational diabetes, starting from before conception until the time of delivery, should be the ultimate goal in order to achieve optimal maternal, foetal and neonatal outcomes.