Introduction
Since December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak, which was first reported in Wuhan, China, has become a serious threat to public health [1]. Later, coronavirus disease 2019 (COVID-19) was declared as a global health emergency by the World Health Organization (WHO) on 11 March 2020, defining it as a pandemic disease [2].
Currently, SARS-CoV-2 infection is so widespread that it will probably become endemic in the human population. In fact, the virus continues to evolve and to adapt; its circulation in a population, either naive or with suboptimal protection from infection, increases the possibility of generating variants with increased transmissibility and resistance to host immunity.
Although most patients infected with SARS-CoV-2 exhibit respiratory symptoms, other organs may also be involved, including the liver, often resulting in liver injury [3]. Liver injury associated with COVID-19 is defined as any liver damage that occurs during the course and treatment of individuals with COVID-19, with or without pre-existing liver disease [4–6].
Most patients with moderate to severe forms of COVID-19 have metabolic comorbidities such as diabetes mellitus and obesity [7]. These conditions are risk factors for the development of non-alcoholic fatty liver disease (NAFLD) [8, 9]. NAFLD is the most common chronic liver disease in the world, and its prevalence is expected to increase together with the epidemics of type 2 diabetes and obesity [10].
Data about liver injury during COVID-19 are numerous, while overviews of this infection in patients with NAFLD, both in respiratory and liver terms, are emerging.
In this review, we summarise the current research focusing on COVID-19 in NAFLD patients. In the first part of review, we discuss the epidemiology and the general mechanisms of liver injury; in the second part, we report clinical evidence on COVID-19 in NAFLD and the association between liver injury in COVID-19 subjects with non-alcoholic fatty liver disease.
Epidemiology of nonalcoholic liver disease
NAFLD is a condition characterized by steatosis in > 5% of the liver parenchyma, in association with metabolic alterations (mainly obesity and type 2 diabetes), without any chronic liver disease, and with ethanol intake not exceeding 30 g/day for men and 20 g/day for women [11]. Recently an international panel of experts proposed renaming NAFLD as metabolic-associated fatty liver disease (MAFLD), giving importance to metabolic syndrome diagnostic criteria rather than alcohol withdrawal [12].
We will use the nomenclature NAFLD in this review. NAFLD can progress to non-alcoholic steatohepatitis (NASH), a condition characterized by steatosis, inflammation, and cell damage.
NAFLD is the leading cause of hepatocarcinoma (HCC) and liver transplantation [13]. NAFLD is the most prevalent chronic liver disease worldwide, and its prevalence ranges from 13.5% in Africa to 31.8% in the Middle East, consistent with differences in genetic predisposition, caloric intake, physical activity, body fat distribution, and socio-economic status [14]. In Italy, the prevalence of NAFLD is 22.5–27% in the general population [15–18]. The prevalence increases with age and is higher in men than in women, particularly in the pre-menopausal period [19, 20]. In addition, ethnic disparities within the prevalence of NAFLD were observed. The prevalence of NAFLD and NASH are highest in Hispanics, intermediate in whites, and lower in blacks, but the underlying causes are unclear [21–23].
The prevalence of NAFLD is additionally increasing in children and young adolescents [24].
NAFLD is diagnosed in 47.3%-63.7% of patients with type 2 diabetes and in up to 80% of obese people [25, 26]. NAFLD and NASH can even be found in lean individuals, particularly within the Asian population [27, 28]. In Italy, NAFLD is reported in 59.0–73.2% of persons with type 2 diabetes [29, 30], and about 13–18% of them have advanced fibrosis [31].
A vicious circle exists between type 2 diabetes and NAFLD [32, 33].
The presence of type 2 diabetes mellitus (T2DM) increases the risk of progression of NAFLD to advanced fibrosis and cirrhosis, as well as incident hepatocellular carcinoma (HCC), liver-related hospital admissions, and liver-related deaths [32, 34, 35] while the presence of NAFLD in T2DM is associated with a reduced likelihood of achieving good glycaemic control, and it aggravates atherogenic dyslipidaemia, further increasing the risk of chronic kidney disease and adverse CV outcomes [32, 36] particularly in the presence of NASH fibrosis [37].
Patients with NAFLD are more likely to develop liver damage when infected with SARS-CoV-2 [38].
Epidemiology of COVID-19
COVID-19 was declared a global pandemic by the WHO in March 2020, because cases were being reported on all continents [39]. To date, more than 609 million people were infected with 6,507,002 deaths worldwide [40]. In Italy, there have been 22,169,273 confirmed cases of COVID-19 with 176,609 deaths [40].
The severity of COVID-19 is related to increased age, male sex, and pre-existing medical diseases [41, 42]. Severe COVID-19, defined as intensive care unit admission or hospitalization, mechanical Ventilation, or death, is associated with underlying diseases such as diabetes mellitus and obesity [43, 44].
Type 2 diabetes and obesity do not increase the risk of contracting COVID-19 infection [45] but may worsen the severity of the disease [44, 45].
Pathogenesis of liver injury during SARS-CoV-2 infection in NAFLD
The specific underlying mechanisms of liver injury in COVID-19 remain undetermined and not fully understood, but emerging data from small clinical case studies have proposed that liver injury in COVID-19 is frequently multifactorial [46].
Below we report literature evidence about this topic. According to the authors, in some assumptions we can suggest a reciprocal link between NAFLD, SARS-CoV-2, and liver injury.
Direct viral effect
It has been demonstrated that SARS-CoV-2 enters host cells through binding of its S protein to angiotensin-converting enzyme 2 (ACE2) on the surface of the host cell [47]. However, based on single-cell sequencing and animal model analysis of liver tissue, Chai et al. found that the expression level of ACE2 in liver tissue was only approximately 0.31% and that specific expression of ACE2 in bile duct epithelial cells was 20 times higher than that in hepatocyte [48].
After SARS-CoV-2 infection, ACE2 expression may be upregulated in hepatocytes as a compensatory response which could further enhance the deleterious effect of SARS-CoV-2 on hepatocytes [49].
Data about ACE2 expression in NAFLD are conflicting. Biquard et al. showed that NAFLD is unrelated to the change in liver expression of COVID-19 infection-related genes, which does not support increased hepatic uptake of SARS-CoV-2 [50], while Meijnikman et al. reported increased hepatic ACE2 expression in patients with NAFLD [51].
Recent studies discuss how the impact of cholestatic injury and cholangiocyte activity/ductular reaction during SARS-CoV-2 infection influences NAFLD progression [52]. While NAFLD is typically considered a disorder of hepatocytes, the underlying role of other liver cell types, including cholangiocytes, is becoming more known. For instance, NAFLD patients with cholestasis have more advanced histological impairments, including cholangitis, advanced fibrosis, and cirrhosis, when compared with age- and sex-matched NAFLD cohorts [52]. Consequently, we could speculate that infection of SARS-CoV-2 can be a risk factor for liver damage in NAFLD patients.
Antibody-dependent enhancement
In addition to ACE2 receptor-mediated viral infection, antibody-dependent enhancement of infection (ADE) may occur in patients with SARS [53]. This mechanism refers to the interaction of a virus-specific antibody with Fc receptor (FcR) and/or complement receptor (CR) to enhance the ability of the virus to enter granulocytes, monocytes, and macrophages.
The virus continuously replicates in the above cells, aggravating the infection [54]. Wang et al. found that antibodies to the SARS-CoV spike protein trigger ADE, causing SARS-CoV to enter immune cells that do not express ACE2 and immune damage [55].
The cytokine storm
An early elevation of several inflammatory cytokines (e.g. interleukin (IL) 6, IL-10, IL-2, interferon (IFN) λ (IFN-λ)) has been found in the vast majority of patients with COVID-19 [56, 57].
This over-response of cytokines against the virus becomes a cytokine storm in the course of the disease in severe cases of COVID-19 [58].
Patients with severe outcomes showed increased levels of inflammatory cytokines IFN-λ, transforming growth factor α, thymic stromal lymphopoietin, IL-16, IL-23, IL-33, and coagulopathy-related mediators such as thrombopoietin [56].
This type of reaction, known as “cytokine storm syndrome”, may be associated with multiorgan failure and disseminated intravascular coagulation due to a dysregulated release of pro-inflammatory cytokines [59, 60].
It is not yet clear whether NAFLD can aggravate or precipitate the cytokine storm. Dysregulated hepatic innate immunity in NAFLD patients could contribute to the pathogenesis of COVID-19. In fact, hepatic innate immunity cells produce active cytokine. NAFLD and obesity are associated with high production of pro-inflammatory cytokines by adipocytes and Kupffer cells [61].
Ischaemia injury
Ischaemia-reperfusion injury during respiratory failure or sepsis is mostly complementary to the previously mentioned dysregulated and uncontrolled immune system. In cases of severe presentation of COVID-19 when systemic inflammatory response syndrome (SIRS) occurs, the uncontrolled release of proinflammatory cytokines causes peripheral vessel vasodilation with reduced blood pressure and generalized tissue hypoxia. If acute respiratory distress syndrome (ARDS) occurs simultaneously, it leads to poor blood oxygenation, which aggravates liver ischaemia.
Under shock and hypoxia, reactive oxygen species (ROS) increase, leading to greater peroxidation of lipids, DNA, and proteins. These peroxidation products and ROS itself activate hepatic stellate cells to produce the extracellular matrix and innate immune cells of the liver, which produce proinflammatory cytokines, leading to further liver damage [62].
Drug-induced liver injury
Given the fact that the liver is involved in the metabolism of many drugs, hepatotoxicity during SARS-CoV-2 infection can arise. In fact, these drugs can include acetaminophen, non-steroidal anti-inflammatory drugs, tocilizumab, methylprednisolone, and remdesivir [63–65].
Several experimental and clinical investigations have hypothesized that obesity and NAFLD may increase the risk of hepatotoxicity of different drugs [66, 67].
COVID-19 in the setting of NAFLD
Among cases with chronic liver diseases and COVID-19, the relationship between NAFLD and COVID-19 has been the most studied. Below we report main evidence.
Prevalence of NAFLD in COVID-19 patients
In the first meta-analysis of 4 studies [68–71] Pan et al. [72] reported a pooled prevalence of 0.31 (95% CI: 0.28–0.35) of NAFLD among COVID-19 patients. Similarly, Hayat et al. [73] in a meta-analysis of 16 studies found a pooled prevalence of COVID-19 among NAFLD patients of 0.29 (95% CI: 0.19–0.40; p < 0.001).
COVID-19 severity in patients with NAFLD
Ji et al. [70], in a retrospective study including 202 patients with COVID-19, reported an association between the progression of COVID-19 disease and older age (> 60 years), male sex, higher body mass index, NAFLD, and higher percentage of comorbidity. In particular, patients with NAFLD had a higher risk of disease progression (6.6% vs. 44.7%; p < 0.0001) and longer viral shedding time (17.5 ±5.2 days vs. 12.1 ±4.4 days; p < 0.0001) compared to patients without NAFLD. The authors reported that NAFLD is an independent risk factor for COVID-19 disease progression [70].
Zheng et al. [71], in a study that included 45 obese and 21 non-obese patients with COVID-19, found a 6-fold increased risk of severe COVID-19 in the presence of obesity in NAFLD patients in the logistic regression model, as well, after adjustment for age, sex, smoking, diabetes, hypertension, and dyslipidaemia.
Targher et al. [74] conducted a cohort study including 310 patients with COVID-19. Hepatic steatosis was defined by computed tomography, fibrosis-4 (FIB-4) index, and NAFLD fibrosis score (NFS) were used to categorie liver fibrosis probability as low, intermediate, or high. The authors reported that the severity of COVID-19 disease was increased in NAFLD patients with elevated or intermediate fibrosis index-4 scores. The authors highlighted study limitations such as small sample size, homogeneous ethnicity of the study population, and lack of histopathology diagnosis of hepatic fibrosis.
In multicentric retrospective study that included 327 adult patients, Zhou et al. showed that NAFLD patients younger than 60 years of age had more severe COVID-19 disease compared to patients older than 60 years of age, independent of other risk factors that increase the severity of COVID-19 [68].
Gao et al. [75], in a retrospective cohort study that included 65 COVID-19 patients with NAFLD and 201 COVID-19 patients without NAFLD, showed that NAFLD was associated with a 4-fold increased risk of COVID-19 serious.
In multicentric retrospective study, Hashemi et al. reported significantly higher rates of ICU admission and the need for mechanical ventilation in patients with NAFLD [76]. Another retrospective study from Israel reported that NAFLD patients were more likely to have severe COVID-19 disease [77].
A retrospective study of more than 6700 COVID-19 patients showed that in hospitalized COVID-19 patients, the history of NAFLD/NASH, determined on the basis of electronic medical record data, was correlated with the increase in COVID-19 hospitalization (OR = 1.86; 95% CI: 1.43–2.42, p < 0.01) [78]. After adjusting for history of NAFLD/NASH, the probability of hospitalization was significantly decreased in obese patients with COVID-19 [78].
A retrospective study of 193 patients with COVID-19 [79] showed that the presence of hepatic steatosis was unrelated to ICU admission (OR = 1.14 [0.53–2.5]) or hospitalization mortality (OR = 0.86 [0.44–1.69]) after adjustment for confounding factors (males, age, hypertension, dyslipidaemia, and DM2).
In another retrospective study on 320 COVID-19 patients with NAFLD and 296 COVID-19 patients without NAFLD, the authors reported that NAFLD was not an independent predictor of mortality or disease progression, contrary to the results of other studies [80].
Tripon et al. [81], in a retrospective study, evaluated 719 COVID-19 patients. Among these, 445 patients were affected by NAFLD. Notably, 311 patients with NAFLD had a mild to moderate form of COVID-19, while a further 134 patients with NAFLD had a severe form of COVID-19. NAFLD was defined as hepatic steatosis index and severity related to the stage fibrosis with NAFLD fibrosis score (NFS). The authors reported that a higher NAFLD fibrosis score was associated with a higher risk of hospitalization (OR = 1.754; 95% CI: 1.27–2.43, p < 0.001) and in multivariate analyses, patients with high fibrosis index-4 were found to have a 3-fold higher risk of severe disease (p < 0.001) [81].
Although some studies have reported that negative outcomes are similar in COVID-19 patients with and without NAFLD, a systematic review specifically assessed NAFLD as a risk factor in patients with COVID-19 and revealed pooled OR for severe COVID-19 in NAFLD [82] while tree systematic reviews with meta-analysis looked several studies to conclude that NAFLD was associated with increased risk of severe COVID-19 and ICU admission [73–84]
In summary, many studies highlight the role of NAFLD in the progression of COVID-19, but there is still no evidence that NAFLD will affect the prognosis of COVID-19 [85].
Liver injury in COVID-19 subjects with non-alcoholic fatty liver disease
COVID-19-associated liver injury should be defined as alanine transaminase (ALT) or asparagine transaminase (AST) exceeding 3 times the upper limit of the normal value and alkaline phosphatase (ALP), glutamyl transferase (GGT), or total bilirubin (TBIL) exceeding 2 times the upper limit of the normal value [86].
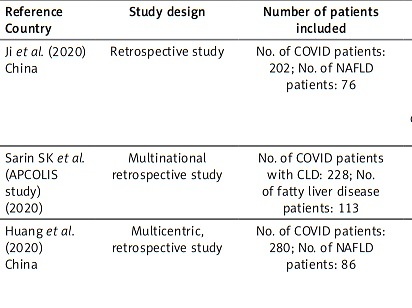
The incidence of liver injury in COVID-19 patients is highly variable, ranging from 15% to 53% [70, 87]. In this section of the article, we will look at specific reports from countries around the world describing the association between liver injury in COVID-19 subjects and non-alcoholic fatty liver disease. Table I shows the principal characteristics of the included studies.
Table I
Characteristics of studies describing the association between liver injury in COVID-19 subjects and non-alcoholic fatty liver disease
In a retrospective study by Ji et al. [70], 202 COVID-19 patients with NAFLD were studied. NAFLD was defined as hepatic steatosis index > 36 and/or US. Liver injury was found in 101 (50%) and 152 (75.2%) patients on admission and during hospitalization, respectively [70].
Patients with NAFLD had a higher likelihood of liver dysfunction from admission to discharge (70% vs. 11.1%; p < 0.0001) compared to patients without NAFLD [70].
The authors hypothesized that in NAFLD patients, the polarization state of liver macrophages could be distorted from inflammation-promoting M1 macrophages to inflammation-suppressing M2 macrophages, leading to the progression of COVID-19 [88].
In a multinational retrospective study by Sarin et al. 228 COVID-19 patients were enrolled. A total of 113 (61%) patients had NAFLD [89]. Liver biopsy or transient elastography from the last 6 months were used to defined NAFLD. NAFLD was the commonest cause for CLD without and didn’t increase the risk of liver injury [89].
In a multicentre retrospective study by Huang et al. 86 COVID-19 patients with NAFLD and 194 COVID-19 patients without NAFLD were studied. NAFLD was defined as hepatic steatosis index (HIS) [38]. NAFLD patients have higher risk of developing liver injury, although liver enzyme levels were not generally high at admission or during hospitalization, but no patient developed severe liver-related complications during hospitalization [38].
In a retrospective study from Qatar [80], 320 COVID-19 patients with NAFLD and 296 COVID-19 patients without NAFLD were studied. NAFLD was defined as hepatic steatosis index.
The authors reported that NAFLD was a predictor of the development of mild liver injury (OR = 2.99; 95% CI: 1.62–4.37; p < 0.001) and moderate liver injury (OR = 5.104; 95% CI: 3.21–6.99; p < 0.001), but NAFLD was not an independent predictor of mortality or disease progression, in contrast to Ji’s study results [80].
A retrospective study from Brasil [90] that included 204 COVID-19 patients (case group) and 112 RT-PCR-negative patients (control group) reported that the incidence of hepatic steatosis in the COVID-19 patients was 4.7 times higher than that in the negative control group (OR = 4.698; 95% CI: 2.12–10.41, p < 0.001).
In a retrospective single-centre cohort study from the USA [91], 342 COVID patients and 178 hepatic steatosis patients were studied. Hepatic steatosis was defined by either imaging evidence of steatosis > 30 days before COVID-19 diagnosis, or hepatic steatosis index (HSI) > 36 for Asians and > 39 for non-Asians. Hepatic steatosis was associated with increased disease severity and transaminitis in COVID-19, but liver-related complications were low and had no association with hepatic steatosis. A limitation of this study was the use of a surrogate measure of HS [91].
In a retrospective study from Mexico [92], 86 COVID-19 patients with NAFLD and 194 COVID-19 patients without NAFLD were studied. NAFLD was defined as hepatic steatosis index (HIS).
The NAFLD patients had a higher risk of developing liver injury, although liver enzyme levels were not generally high at admission or during hospitalization, but no patient developed severe liver-related complications during hospitalization [92].
In another retrospective single-centre cohort study by Younossi et al. [93] 553 COVID-19 patients with NAFLD and 2736 COVID-19 patients without NAFLD were studied. Magnetic resonance imaging, computer tomography, or ultrasound were used to defined NAFLD. Of the patients with NAFLD, 3.9% had acute liver injury recorded during their stay (vs. 1.6% in non-NAFLD controls; p = 0.0006). The patients with NAFLD required greater use of hospital resources. Independent predictors of mortality included higher FIB-4 and multimorbidity scores, morbid obesity, older age, and hypoxaemia on admission [93].
Implications of COVID-19/NAFLD co-prevalence and COVID-19 pharmacological treatment
NAFLD is the most common chronic liver disease in the world [14], and it is hypothesized to be present in the majority of COVID-19 patients with pre-existing CLD worldwide [94].
General recommendations for people with NAFLD are similar to those of the general population, including use of masks, vaccination, thorough hand washing, social distancing, increased personal protection, good coughing habits, and avoidance of sick people [95].
The pandemic has led to greater adoption of unhealthy lifestyles and an increase in the prevalence of obesity, which can drive the development and progression of NAFLD [96].
Patients with NAFLD may have other metabolic disorders such as obesity, type 2 diabetes mellitus, and hypertension, which may increase risk of a severe outcome of COVID-19 [97, 98].
Proper lifestyles (including recommendations for weight loss, regular physical activity), and monitoring and adequate treatment of chronic diseases such as type 2 diabetes mellitus and hypertension could reduce the risk of contracting COVID-19 infection as well as the risk of severe cases [95].
The optimal approach to the management of patients with liver chronic disease who acquire SARS-CoV-2 infection is still evolving [99], and it is no different from patients without liver disease [100].
In mild to moderate cases of COVID-19 that usually do not require hospitalization, in addition to symptomatic therapy, antiviral drugs (remdesivir, nirmatrelvir/ritonavir, molnupiravir) and monoclonal antibodies (casirivimab/imdevimab, bamlanivimab/etesevimab, and sotrovimab) may be indicated [101].
In severe cases of COVID-19, which typically require hospitalization, corticosteroids (dexamethasone), anticoagulant therapy with low-molecular-weight heparin (LMWH), and oxygen therapy are recommended. In patients who do not improve with conventional oxygen therapy, high-flow oxygen or mechanical ventilation are indicated [102, 103].
Monoclonal antibodies and antiviral drugs are indicated in selected cases [102].
The AASLD guidelines recommend regular monitoring of liver function in all hospitalized COVID-19 patients [104].
Also for patients with NAFLD, the best strategy is the prevention of infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and attenuation of coronavirus disease 2019 (COVID-19).
Preliminary data indicate that vaccination in NAFLD patients without cirrhosis is safe and associated with a good humoral response [105].
Strengths and limitations
This review thoroughly analysed the relationship between liver injury in COVID-19 subjects and non-alcoholic fatty liver disease.
There are also several limitations to this review. The studies included in this review are retrospective studies, which may have been influenced by confounding factors such as differences in diagnostic criteria, small sample size, and limited survey area.
Furthermore, liver biopsies were obviously not performed in most of the studies, and in particular there are few data on the correlation between COVID-19 and the long-term progression of liver disease, which does not allow an assessment of the causal link between NAFLD and the risk of infection and progression of COVID-19.
Conclusions
After the lung, the liver is the most common organ affected by COVID-19. Some studies have shown that NAFLD is associated with severe COVID-19 and with poor results; however, other studies did not show significant differences between groups in comparing complications and clinical outcomes. Patients with NAFLD can suffer from severe COVID-19 due to other comorbidities, particularly cardiovascular disease.
Furthermore, other mechanisms such as an increased susceptibility to infections, a reduced immune Response, and an increased risk of coagulation could contribute to a higher risk of severe COVID-19 in people with NAFLD [106].
Liver injury due to COVID-19 infection in NAFLD patients is probably multifactorial, e.g. potentially hepatotoxic drug use, systemic inflammatory response, respiratory distress syndrome-induced hypoxia, and direct injury.
Liver involvement in NAFLD and COVID-19 patients appears to be only mild to moderate, which questions its clinical significance in COVID-19. Because COVID-19 is a new disease, more prospective or larger retrospective studies are needed to better understand the behaviour of the virus and its interaction with other diseases.