Introduction
Congenital heart diseases (CHD) are the most common birth defects, with an approximate incidence of 0.8% [1]. Since the first repair of congenital cardiac defects in the 1950s, the overall mortality rate has decreased to less than 5% in different types of congenital cardiac defects [2]. Those operations are also widely performed in Turkey with certain success rates.
Cardiopulmonary bypass (CPB) or extracorporeal circulation is an inevitable component of cardiac surgery procedures. It temporarily replaces the heart and the lungs to perfuse the body for a bloodless, steady and comfortable field to facilitate the procedure and to assist the surgeon. Despite the technologic advances in the medical field, CPB has never become physiologic and carries certain hazards. The most important complication related to CPB is secondary to tissue oxygenation. The literature includes many studies aimed at reducing the complications of CPB.
Ischemic and hypoxic changes occur especially in tissues, such as heart, lungs, brain, kidneys, gastrointestinal system and liver during CPB, which increase the morbidity and mortality rates during the postoperative period [3]. Monitoring of somatic and cerebral oxygenation during CPB has proven importance to reduce and prevent neurological and somatic damage. Tissue oxygenation may be assessed with heart rate, arterial oxygen saturation, lactate levels and mean arterial pressure; however, these standard monitors may not always be precise or sufficient [4]. Additional precautions to test the tissue perfusion with side-stream dark field microscopy, capillaroscopy, video Doppler capillaroscopy, laser Doppler flowmetry, thermography or transcutaneous oxygen measurement may also be used [5]; however, they are not practical. The easily applicable and widely available near-infrared spectroscopy (NIRS) is necessary in many cardiac surgery procedures and benefits of the use of NIRS for cerebral and somatic oxygenation during cardiac surgery have been proven in the literature [6, 7].
The literature lacks comparisons of NIRS values in cyanotic and acyanotic pediatric patients who undergo cardiac surgery. The aim of this study was to evaluate the factors affecting bilateral cerebral and somatic NIRS values in patients with congenital cardiac defects, as well as to compare the NIRS values between cyanotic and acyanotic groups.
Material and methods
The prospective study included consecutive patients aged between 0 and 60 months and having congenital cardiac defects who required cardiac surgery between August 2016 and January 2017. The research was conducted following necessary approval from the institutional ethics committee. The patients were enrolled in the study following their parents’ consent after being informed in detail about the protocol.
Patients were divided into two groups as having a cyanotic congenital cardiac defect and possessing acyanotic congenital cardiac lesions. Each group consisted of 15 patients. Patients with severe heart failure, multiple organ failure, neurological sequela or syndromes, or active infection were excluded from the study.
Patients’ length, weight, body mass indices, laboratory findings including complete blood count and biochemistry values (liver, kidney and thyroid functions, electrolytes, glucose levels) were assessed. Echocardiography, computed tomography angiography and/or cardiac catheterizations were recorded. Cardiopulmonary bypass cannula sizes and perfusion flow values were adjusted according to the body surface area of each patient.
Data were collected at anesthesia induction (T1), at the 10th min of CPB (T2), 30th min of CPB (T3), every 30 min during CPB (T4, T5, T6, T7, …etc.) and 1 h after CPB (TS). The NIRS (right and left cerebral hemispheres, right and left somatic regions), mean arterial pressure, arterial blood gas analysis (pH, partial carbon dioxide pressure (pCO2), partial oxygen pressure (pO2), hematocrit, lactate, bicarbonate (HCO3) and base excess values), CPB flow (excluding T1 and TS), CPB and cross clamp times, and heat exchange values were recorded for further analysis.
Anesthesia protocol
Anesthesia was started with 5 mg/kg of intramuscular ketamine 30 min after 0.5 mg/kg oral midazolam premedication. The intravenous anesthesia was performed with 10 µg/kg of fentanyl, 0.1 mg/kg of vecuronium bromide and 0.2 mg/kg of midazolam. Patients’ lungs were ventilated with oxygen, air, and isoflurane, and ventilation was adjusted to maintain PaCO2 of 30–40 mm Hg. Maintenance of the general anesthesia was achieved with infusion of 5 µg/kg/h of fentanyl and sevoflurane titrated in 0.5% to 2% inspiratory concentration of 50% oxygen and 50% of H2O to keep the mean arterial pressure and heart rate within ±20% of normal values. Additional doses of 2 µg/kg fentanyl, 0.1 mg/kg midazolam and 0.025–0.05 mg/kg vecuronium bromide were injected when required. Noninvasive monitoring consisted of electrocardiogram, pulse oximetry, and measurements of inspiratory and expiratory gas concentrations, and invasive monitoring was achieved with central venous and radial/femoral arterial lines. All the patients received 5% 0.45 NaCl solution at a rate of 10 ml/kg/h. Patients were ventilated with pressure control mode of the ventilator to achieve 6–8 ml/kg tidal volume, 1/2 ratio of inspiration and expiration with a positive end expiratory pressure in the range 3–5 mm Hg. The ventilatory rate was adjusted to keep the end-tidal CO2 rate in the range 30–40 mm Hg. Body temperature was measured with nasopharyngeal and rectal probes.
Surgical technique
The operations were performed with hypothermic CPB through median sternotomy. After systemic heparinization (activated coagulation time > 480 s), CPB was established through cannulation of the ascending aorta and both caval veins. A non-pulsatile roller pump and disposable membrane oxygenator were used. Oxygenator and tubing sets were chosen depending on the perfusion flows and Terumo Capiox FX 05 and Terumo Capiox FX 10 (Terumo Cardiovascular Group, Ann Arbor, MI, USA) were used when the flow was below 1200 ml and above 1200 ml, respectively with heparin bonded circuits. Tubing sets were connected with 1/4–1/4 connectors in patients up to 16 kg of body weight and with 1/4–3/8 when the patients weighed over 16 kg. Additional safety measures for bubble detection, level sense, pressure module, and a venous heat monitor were connected to the CPB circuits.
Prime volume was adjusted with 2.5 ml/kg mannitol, 1 ml/kg 10% calcium (max. 10 ml), 1 ml/kg NaHCO3 (max. 10 ml), 500 units of heparin for each 100 ml of prime volume, balanced electrolyte solution (isolyte-S), human albumin at a dose of 2 ml/kg when the patient had hypoalbuminemia, antibiotic solution (cefazolin 20 mg/kg), fresh frozen plasma and erythrocyte suspension with the aim to keep the hematocrit level around 30% during perfusion. Systemic temperature was kept between 27° and 32°C during cardiac arrest. Flow rates were maintained at 2.8 l/min/m2 at 37°C, 2.4 l/min/m2 at 32°C, and 2.2 l/min/m2 at 28°C to maintain a venous oxygen saturation between 70% and 90% and mean arterial pressure depending on the pathophysiology of the underlying diseases. CPB was initiated with low oxygen saturation (70–80%) in patients with cyanotic congenital heart diseases. Oxygen concentration was gradually increased to desired levels. Myocardial protection was achieved with intermittent cold blood cardioplegia. A dose of 20 ml/kg of cold blood cardioplegia was initially infused into the aortic root at a pressure of 100 mm Hg to achieve cardiac arrest, with subsequent doses of 15 ml/kg every 15 min. Hypothermic circulatory arrest was not required in any of the cases. Weaning off CPB was commenced at a rectal temperature of 37°C. Infusion of milrinone, dopamine, dobutamine, adrenalin and/or noradrenalin was provided without hesitation when needed. Modified or conventional ultrafiltration was performed if required.
Application of NIRS
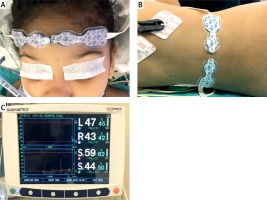
Near infrared spectroscopy measurements were performed with INVOS Cerebral/Somatic Oximeter (Medtronic, Somanetics, Covidien, Mansfield, MA 02048 USA). Right and left cerebral hemispheric probes were attached to the forehead (Figure 1 A). Somatic probes were attached to the back of the patient around the anatomical projections of the right and left kidneys (Figure 1 B). Cerebral and somatic NIRS values were measured simultaneously (Figure 1 C). Each data set was separately studied for right and left hemispheres and right and left somatic regions.
Figure 1
A – Projection of the forehead – right and left frontal lobes of the patient. B – Lumbar projection – right and left renal region of the patient. C – Simultaneous measurement of bilateral cerebral and somatic NIRS with INVOS Cerebral/Somatic Oximeter (Medtronic, Somanetics, Covidien, Mansfield, MA 02048 USA)
Statistical analysis
NCSS (Number Cruncher Statistical System) 2007 (Kaysville, Utah, USA) computer software was used for statistical analysis. Descriptive data were presented as frequency, ratio, minimum, maximum, median, ± mean standard deviation. Quantitative data were compared between two groups using the Mann-Whitney U test to compare the quantitative data of 2 groups in which the variables did not show normal distribution. The Friedman test was applied in group comparisons of non-parametric repeated variables. The Bonferroni corrected Wilcoxon signed ranks test was used to evaluate binary comparisons. The Pearson χ2 test was used to compare qualitative data. The significance was evaluated at the level of p < 0.01 and p < 0.05.
Results
The study was carried out with 30 patients who underwent congenital cardiac surgery at our institution (B.A.H.) between August 2016 and January 2017. There were 12 (40%) girls and 18 (60%) boys. The mean age of the patients was 21.29 ±19.33 months (range: 0.70–60.83 months). The age of the patients in the cyanotic group ranged between 0.70 and 42.5 months (mean: 16.27 ±14.54 months) and in the acyanotic group between 1.0 and 60.83 months (mean: 26.31 ±22.54 months). Median weights and lengths of the patients in the cyanotic and acyanotic groups were 8.5 (range: 5–12.6) kg vs. 9.5 (range: 5.3–13) kg and 68 (range: 59–97) cm vs. 85 (range: 61–103) cm, respectively. There was no statistically significant difference in terms of age, weight, height, and gender distribution between the two groups. The demographic data of the patients are presented in Table I for the cyanotic group and Table II for the acyanotic group. All the procedures were carried out at moderate hypothermia values at temperatures between 28°C and 32°C. We did not observe any neurologic dysfunction or visceral or multi-organ failure.
Table I
Demographic features of the cyanotic patients
[i] CC – cross clamp, CPB – cardiopulmonary bypass, TOF – tetralogy of Fallot, ASD – atrial septal defect, APCA – aortopulmonary collateral, DORV – double outlet right ventricle, RV – right ventricle, TGA – transposition of great arteries, PFO – patent foramen ovale, PH – pulmonary hypertension, VSD – ventricular septal defect, PS – pulmonary stenosis, LPA – left pulmonary artery.
Table II
Demographic features of the acyanotic patients
[i] CC – cross clamp, CPB – cardiopulmonary bypass, ASD – atrial septal defect, PH – pulmonary hypertension, VSD – ventricular septal defect, PAPVD – partial anomaly of pulmonary venous drainage, AO – aorta, PDA – patent ductus arteriosus, MS – mitral stenosis, MY – mitral insufficiency, AVSD – atrioventricular septal defect, CAVSD – complete atrioventricular septal defect, AV – atrioventricular.
Evaluation of cerebral NIRS measurements
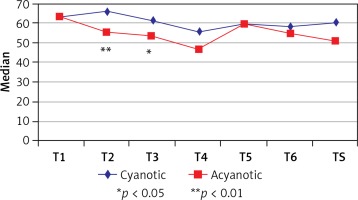
The left cerebral hemispheric NIRS values at all time points in patients in the cyanotic group did not show statistically significant differences. On the other hand, in the acyanotic group the left hemispheric NIRS values indicated a statistically significant decrease at the 30th min of CPB (T3) when compared with the baseline NIRS (T1), which was measured at anesthesia induction (p = 0.028). When we compared the left hemispheric NIRS values of cyanotic and acyanotic groups, the NIRS values were significantly higher in the acyanotic group at the 10th (T2) and 30th (T3) min of CPB (p = 0.009 and p = 0.023). Data regarding the left hemispheric measurements are presented in Table III and Figure 2.
Table III
Evaluation of left cerebral NIRS measurements
| Left cerebral | Cyanotic | Acyanotic | aP-value | ||
|---|---|---|---|---|---|
| Median (Q1, Q3) | Median (Q1, Q3) | ||||
| T1 | 63 (50, 74) | 63 (58, 72) | 0.775 | ||
| T2 | 66 (56, 76) | 55 (47, 61) | 0.009** | ||
| T3 | 61 (56, 72) | 53 (44, 58) | 0.023* | ||
| T4 | 56 (48, 66) | 47.5 (44, 56) | 0.234 | ||
| ‡T5 | 59 (55, 67) | 60 (46, 60) | – | ||
| ‡T6 | 58.5 (49.5, 72.5) | 55 (55, 65) | – | ||
| TS | 60 (48, 67) | 51 (44, 55) | 0.074 | ||
| bP-value | 0.081 | 0.007** | aP-value | ||
| Median (Q1, Q3) | cP-value | Median (Q1, Q3) | cP-value | ||
| T2-T1 | 3 (–3, 9) | 0.928 | –8 (–17, 0) | 0.063 | 0.002** |
| T3-T1 | 5 (–2, 11) | 0.999 | –11 (–17, –4) | 0.028* | 0.004** |
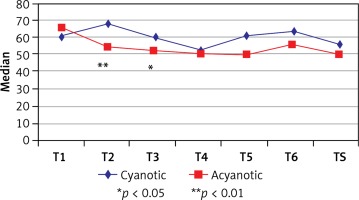
The right hemispheric NIRS values of the cyanotic group indicated a statistically significant decrease at the 60th min of CPB (T4) when compared with the 10th (T2) and 30th (T3) min of CPB (p = 0.015 and p = 0.042), whereas there was a statistically significant decrease of right hemispheric NIRS values at anesthesia induction (T1) and T2 (10th min of CPB), T3 (30th min of CPB) and TS (1 h after CPB) in the acyanotic group (p = 0.007, p = 0.012, and p = 0.045). When we compared the cyanotic and acyanotic groups, the NIRS values measured at T2, T3 and TS were significantly lower in the cyanotic group than the acyanotic group (p = 0.007, p = 0.012, and p = 0.045). Data and statistical analysis values regarding the right hemispheric measurements are presented in Table IV and Figure 3.
Table IV
Evaluation of right cerebral NIRS measurements
| Right cerebral | Cyanotic | Acyanotic | aP-value | ||
|---|---|---|---|---|---|
| Median (Q1, Q3) | Median (Q1, Q3) | ||||
| T1 | 61 (46, 75) | 66 (57, 72) | 0.389 | ||
| T2 | 68 (53, 72) | 54 (47, 62) | 0.005** | ||
| T3 | 61 (50, 66) | 52 (46, 55) | 0.011* | ||
| T4 | 53 (45, 65) | 50.5 (47, 58) | 0.354 | ||
| ‡T5 | 61.5 (55, 69) | 50 (42, 51) | – | ||
| ‡T6 | 63 (53, 69) | 56 (53, 60) | – | ||
| TS | 56 (47, 69) | 50 (47, 56) | 0.116 | ||
| bP-value | 0.001** | < 0.001** | aP-value | ||
| Median (Q1, Q3) | cP-value | Median (Q1, Q3) | cP-value | ||
| T2-T1 | 6 (–3, 9) | 0.776 | –9 (–16, –3) | 0.007** | < 0.001** |
| T3-T1 | 3 (–5, 12) | 0.999 | –13 (–17, –6) | 0.012* | 0.001** |
The decrease in NIRS values at T2 and T3 time points were attributed to decreasing body temperatures during the procedure. We further assessed the risk factors which may affect the changes in the left and right cerebral NIRS values with regression analysis. We evaluated the differences in two categories as the differences between T1 (anesthesia induction) and T2 (10 min after CPB) and the differences between T1 and T3 (30 min after CPB).
The pCO2, mean arterial pressure, flow rates, and lactate values did not influence the left cerebral NIRS difference significantly at T1 and T2 with regression analysis. Additionally the linear regression model was not statistically significant at the mentioned time points (F = 0.614; p = 0.657). On the other hand, mean arterial pressure and lactate values affected the left cerebral NIRS difference significantly at T1 and T3 with regression analysis (F = 4.824; p = 0.005), whereas the flow rates and pCO2 did not have a significant influence on the left cerebral hemispheric NIRS values.
Similarly, the pCO2, mean arterial pressure, flow rates, and lactate values did not influence the right cerebral hemispheric NIRS difference significantly at T1 and T2 with regression analysis (F = 1.631; p = 0.199). However, only the mean arterial pressure affected the right cerebral hemispheric NIRS difference significantly at T1 and T3 with regression analysis (F = 2.937; p = 0.041), and lactate, pCO2 or flow rates did not have a significant influence.
Evaluation of the somatic NIRS measurements
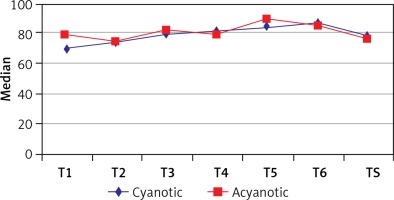
We detected a statistically significant increase in the left somatic NIRS values at T4 and TS when compared with the T1 (p = 0.041, p = 0.011) in the cyanotic group. Moreover, when the left somatic NIRS at T2 was compared with the one at T4 the increase was again statistically significant (p = 0.037) in this group. On the other hand, the left somatic NIRS values did not show a statistically significant difference at any time points in the acyanotic group. Interestingly, the cyanotic and acyanotic groups indicated similar left somatic NIRS values, revealing no statistically significant difference. Data regarding the left somatic NIRS measurements are presented in Table V and Figure 4.
Table V
Evaluation of left somatic measurements according to groups
| Left somatic | Cyanotic | Acyanotic | aP-value | ||
|---|---|---|---|---|---|
| Median (Q1, Q3) | Median (Q1, Q3) | ||||
| T1 | 70 (62, 80) | 78 (66, 84) | 0.174 | ||
| T2 | 74 (68, 79) | 74 (70, 81) | 0.902 | ||
| T3 | 79 (73, 88) | 81 (73, 89) | 0.806 | ||
| T4 | 81 (72, 93) | 78 (68, 85) | 0.252 | ||
| ‡T5 | 83.5 (78, 95) | 89 (84, 90) | – | ||
| ‡T6 | 86 (77, 95) | 85 (84, 89) | – | ||
| TS | 78 (76, 91) | 76 (68, 84) | 0.161 | ||
| bP-value | < 0.001** | 0.317 | aP-value | ||
| Median (Q1, Q3) | cP-value | Median (Q1, Q3) | cP-value | ||
| T2-T1 | 1 (–9, 11) | 0.999 | 2 (–13, 5) | 0.999 | 0.624 |
| T3-T1 | 7 (4, 17) | 0.114 | 8 (–2, 10) | 0.515 | 0.486 |
| T4-T1 | 14 (6, 19) | 0.041* | 5.5 (–10, 11) | 0.999 | 0.063 |
| TS-T1 | 9 (4, 20) | 0.011* | 1 (–10, 14) | 0.999 | 0.106 |
| T3-T2 | 7 (0, 12) | 0.076 | 6 (–5, 14) | 0.723 | 0.683 |
| T4-T2 | 11 (3, 20) | 0.037* | 3.5 (–6, 11) | 0.999 | 0.102 |
| TS-T2 | 9 (–1, 19) | 0.230 | 4 (–10, 12) | 0.999 | 0.202 |
| T4-T3 | 2 (–1, 12) | 0.999 | –0.5 (–12, 5) | 0.999 | 0.158 |
| TS-T3 | 0 (–4, 3) | 0.999 | –2 (–12, 5) | 0.999 | 0.305 |
| TS-T4 | 0 (–10, 0) | 0.999 | 0 (0, 0) | 0.999 | 0.652 |
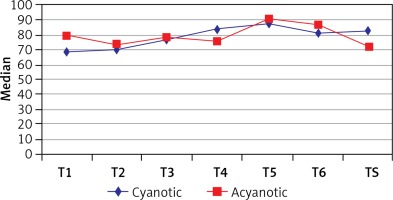
The right somatic NIRS values of the cyanotic group showed a statistically significant increase in values 1 hour after CPB (TS) when compared with anesthesia induction (T1), (p = 0.023). However, as in case of left somatic NIRS measurements, the right somatic NIRS values did not show a statistically significant difference at any time points in the acyanotic group. When we compared the cyanotic group with the acyanotic group, the right somatic values were found to be similar. Data and statistical analysis values regarding the right hemispheric measurements are presented in Table VI and Figure 5.
Table VI
Evaluation of right somatic measurements according to groups
| Right somatic | Cyanotic | Acyanotic | aP-value | ||
|---|---|---|---|---|---|
| Median (Q1, Q3) | Median (Q1, Q3) | ||||
| T1 | 68 (61, 79) | 79 (66, 83) | 0.233 | ||
| T2 | 71 (65, 79) | 74 (70, 78) | 0.595 | ||
| T3 | 76 (72, 88) | 78 (71, 91) | 0.744 | ||
| T4 | 84 (73, 91) | 75 (60, 90) | 0.270 | ||
| ‡T5 | 87.5 (73, 95) | 90 (86, 91) | – | ||
| ‡T6 | 82 (73, 95) | 86 (83, 89) | – | ||
| TS | 82 (71, 88) | 72 (69, 85) | 0.250 | ||
| bP-value | 0.001** | 0.115 | aP-value | ||
| Median (Q1, Q3) | Median (Q1, Q3) | ||||
| T2-T1 | 0 (–9, 20) | 0.999 | –2 (–10, 6) | 0.999 | 0.713 |
| T3-T1 | 7 (1, 20) | 0.325 | 7 (–4, 11) | 0.775 | 0.412 |
| T4-T1 | 12 (3, 25) | 0.082 | 6.5 (–23, 11) | 0.999 | 0.077 |
| TS-T1 | 9 (1, 19) | 0.023* | 5 (–13, 12) | 0.999 | 0.161 |
| T3-T2 | 6 (0, 13) | 0.184 | 5 (–1, 9) | 0.307 | 0.713 |
| T4-T2 | 11 (3, 21) | 0.134 | 3.5 (–8, 8) | 0.999 | 0.085 |
| TS-T2 | 11 (1, 17) | 0.308 | 5 (–8, 8) | 0.999 | 0.089 |
| T4-T3 | 1 (–1, 7) | 0.999 | –1 (–16, 4) | 0.999 | 0.158 |
| TS-T3 | 0 (–7, 2) | 0.999 | –3 (–11, 3) | 0.860 | 0.202 |
| TS-T4 | 0 (–5, 0) | 0.999 | 0 (–1, 0) | 0.999 | 0.847 |
The correlation analysis did not indicate a strong or weak, positive or negative, statistically significant correlation between left and right cerebral and/or somatic NIRS values. On the other hand, increased urine out was associated with increased somatic and cerebral NIRS values.
Discussion
Congenital heart diseases account for the most common birth defects among newborn babies. Since the initial attempts to repair congenital cardiac defects in the 1950s, mortality has decreased dramatically with increasing experience, advancing technology, and surgical and anesthesia techniques. However, neurological and developmental problems may occur in surviving patients and research is continued to avoid these complications.
Monitoring cerebral and somatic oxygen saturation during surgery is important to determine the adequacy of CPB flow and reduce and/or prevent neurological damage, mortality, and morbidity. For this reason, the widely available NIRS device is frequently preferred in recent years. The NIRS device uses probes attached to the surface of the different regions on the body, such as the front of the head or to the back, to the projections over the kidneys, to measure the cortical and renal regional hemoglobin oxygen saturations [8]. The probe emits laser light capable of tissue penetration. The light is reflected and absorbed after contact with hemoglobin molecules depending on the oxygen concentration of the hemoglobin [9].
Vretzakis et al. [8] indicate the normal cerebral oximetry values between 60% and 80%, which may be as low as 55–60% in room air conditions in patients scheduled for cardiac surgery. These values are strongly associated with cardiac output, inspired oxygen saturation, hemoglobin concentration, pulmonary functions, intraoperative cardiovascular collapse, hemorrhage, hypoxemia, arterial inflow or venous outflow occlusion, cerebral temperature and metabolism, acid-base status including pO2, pCO2, etc. [8].
Studies demonstrated the efficiency of the use of NIRS in pediatric cardiac surgery to follow the cerebral oxygenation [6] and in the follow-up of somatic oxygenation [7]. The baseline NIRS measured oximetry values of the brain and the somatic tissues reflect the patient’s cardiopulmonary functions and systemic oxygen needs [8]. Considering that changes in tissue oxygenation may be the cause of complications associated with CPB, it is very well understood that monitoring of tissue oxygenation during CPB has critical importance [10, 11].
In addition to the heart, lungs and central nervous system, ischemic changes in somatic tissues such as the kidney, liver, spleen and gastrointestinal system also occur during open heart surgery, which may lead to morbidity in the postoperative period. As a result, the length of hospital stay and mortality rates increase [3]. It is known that parameters such as arterial oxygen saturation, heart rate and mean arterial pressure, which are used during standard monitorization in cardiac surgery, may not be sufficient to show adequate tissue perfusion and oxygenation [4].
Monitoring of cerebral and somatic perfusion and oxygenation with NIRS provides a more comprehensive evaluation of systemic oxygen demand and systemic oxygen delivery. Bilateral cerebral and somatic NIRS monitoring measures regional oxygen saturation in cerebral and renal (somatic) circulations. In normal conditions, cerebral O2 saturation is approximately 10% less than renal (somatic) O2 saturation [12]. The reason for the difference between somatic and cerebral NIRS is related with metabolism and changes in tissue perfusion [13].
Marimon et al. [14] in their study investigated cerebral and somatic NIRS values and correlated them with venous O2 saturation. They found a significant relationship between the parameters; however, they could not conclude about the best oximetric modality for the determination of cardiac output during the postoperative treatment of these patients [14]. In an another study comparing the NIRS values between cyanotic and acyanotic patients Ersoy et al. [15] emphasized that renal NIRS values were lower in cyanotic patients even after corrective cardiac surgery [15]. Our study supports these findings with significantly lower NIRS values in the cyanotic group than the acyanotic group. Li et al. [16] stressed the importance of oxygen delivery especially in conditions when the neonatal brain required increased oxygen such as during the repair of congenital heart diseases and showed the detrimental effects of the issue on the neonatal nervous tissues.
Vasoconstriction, renal hypoperfusion, somatic regional O2 saturation reduction and consequent loss of pulsatility may lead to progressive hypotension and shock. NIRS application, in contrast to other methods for the determination of tissue oxygenation, is also very useful in severe circulatory shock conditions when there is end-organ perfusion collapse. It is also applicable when the blood flow is non-pulsatile [13, 17]. The reliable applicability of NIRS in non-pulsatile conditions enhanced the use of NIRS especially during CPB or in patients receiving extracorporeal membrane oxygenation therapy at the intensive care units [18].
There are no pre-determined cerebral or somatic thresholds for ischemic conditions in the human body. However, Kurth et al. [19] created a cerebral hypoxemia model in normothermic conditions in neonatal pigs. They measured the baseline cerebral oxygen saturations around 68% and they determined that the cerebral oxygen saturation thresholds for increased lactate, electroencephalography change, and decreased brain energy consumption occurred at levels below 44% [19]. However, there is no available consensus yet and it is very difficult to determine at what threshold values or how large a decrease in the baseline values would be the cause of neurological consequences. In clinical practice, basal values are taken for each patient and precautions are recommended when there is a 20% decrease [20].
Holtby et al. [21] stated that the maintenance of NIRS monitorization in the intensive care unit during the postoperative period will be beneficial for the early diagnosis of perfusion defects due to reasons such as sepsis, bleeding or low cardiac output. The reliability of NIRS has been proven in many studies comparing jugular venous bulb and central venous saturation measured by invasive means [22, 23]. Another study carried out with NIRS showed that cerebral oxygen saturation determined with NIRS was around 70% in acyanotic patients, while it was within the range 40–60% in cyanotic patients at room air conditions [24].
It is proposed that the NIRS values may be affected by various external and internal stimuli during CPB. Those also correlate well with vital findings such as body temperature, perfusion pressure, central venous pressure, and arterial oxygen saturation researched in 10 children undergoing CPB surgery [25]. In our study, mean arterial pressure and lactate values affected the left cerebral NIRS difference significantly at T1 (the baseline NIRS at anesthesia induction) and T3 (30th min of CPB), whereas the flow rates and pCO2 did not have a significant influence on the left cerebral hemispheric NIRS values. The right cerebral hemispheric NIRS difference was significantly affected by only the mean arterial pressure at T1 (the baseline NIRS at anesthesia induction) and T3 (30th min of CPB ). Lactate, pCO2 or flow rates did not have a significant influence.
Smith et al. [26] studied the transfusion requirement in trauma cases. They reported that somatic NIRS was helpful for the decision for transfusion in this particular patient population with high risk of hemorrhagic shock. The tissue oxygen saturations were early markers to predict the need for early blood transfusion in trauma patients in their study [26].
Gottlieb et al. [27], by monitoring bilateral cerebral NIRS, detected a sudden decrease in cerebral oxygen saturations after the initiation of CPB, which was more pronounced in the left cerebral hemispheric NIRS probes than the right side. In contrast, Durandy et al. [28] reported no difference in NIRS values; however, they detected a large parietal-occipital ischemic area in the magnetic resonance imaging studies in their study with pediatric patients undergoing cardiac surgery [28]. In our study, the left cerebral hemispheric NIRS values at all time points in patients in the cyanotic group did not show statistically significant differences. On the other hand, in the acyanotic group the left hemispheric NIRS values indicated a statistically significant decrease at the 30th min of CPB (T3) when compared with the baseline NIRS (T1) which was measured at anesthesia induction. When we compared the left hemispheric NIRS values of cyanotic and acyanotic groups, the NIRS values were significantly higher in the acyanotic group at the 10th (T2) and 30th (T3) min of CPB. The right hemispheric NIRS values of the cyanotic group indicated a statistically significant decrease at the 60th min of CPB (T4) when compared with the 10th (T2) and 30th (T3) min of CPB, whereas there was a statistically significant decrease of right hemispheric NIRS values at anesthesia induction (T1) and T2 (10th min of CPB), T3 (30th min of CPB) and TS (1 h after CPB) in the acyanotic group. The changes in NIRS values at the aforementioned time points were attributed to the temperature decrease. When we compared the cyanotic and acyanotic groups, the NIRS values at T2, T3 and TS were found to be significantly lower in the cyanotic group than the acyanotic group.
The literature lacks a detailed comparison of bilateral cerebral and somatic NIRS values in cyanotic and acyanotic patients who receive open heart surgery for congenital cardiac defects. In the current research we investigated the cerebral and somatic oxygenation in pediatric patients with cyanotic and acyanotic congenital cardiac pathologies with NIRS monitors and the factors that may influence the NIRS values during CPB at different time intervals. However, the NIRS values may be dependent on many different variables. In this study the significant increase in partial oxygen pressure after the initiation of CPB may be the main reason for increased cerebral and somatic NIRS values 10 min after the onset of CPB, which was more pronounced in the cyanotic group when compared with the acyanotic patients. Right-left somatic NIRS values were not significantly different during all measurement times in cyanotic and acyanotic patients. The NIRS values of cyanotic cases were significantly higher in the left cerebral measurements at the T2 and T3 time points in the acyanotic patients. Regression analysis revealed that statistically significant effects were attributed to the changes in the mean arterial pressure and lactate levels at T1 and T3 and the difference in the right and left cerebral hemispheres. Although the cerebral NIRS values in the acyanotic group decreased, the reason for the stable monitoring of somatic NIRS values may be related to regional perfusion, metabolism differences and systemic vascular resistance regulation of somatic organs.
A major limitation of the study is the relatively small sample sizes. The cohorts are also not homogeneous, meaning comprising patients with versatile congenital cardiac pathologies. Another limitation regards the patient specific factors such as skull and fat thickness projection over the kidney, which might influence the NIRS measurements; however, we relied on preoperative baseline values and gradients during CPB. Another limitation regards the age of the cohort, which included patients up to 60 months of age.
In conclusion, the use of advanced monitoring techniques to reduce morbidity and mortality in pediatric and congenital cardiac surgery has become increasingly important. NIRS acts as an immediate indicator for events during cardiopulmonary bypass and alerts for rapid action for adequate tissue perfusion, since cerebral and somatic NIRS monitorizations are non-invasive, cost-effective and instant-continuous methods. The routine use during congenital cardiac surgery, or at the intensive care unit during the follow-up may be recommended for enhanced evaluation of the tissue oxygenation. The results of the current research suggest that attempts for higher CPB flow, increased hematocrit and pO2 levels could be implemented for adequate cerebral and somatic perfusion pressures to keep NIRS values higher during operations on cyanotic and acyanotic patients undergoing congenital cardiac surgery.